MitraphyllineCAS# 509-80-8 |
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Uncarine A
Catalog No.:BCN7767
CAS No.:6899-73-6
- Uncarine F
Catalog No.:BCN9446
CAS No.:14019-66-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 509-80-8 | SDF | Download SDF |
| PubChem ID | 94160 | Appearance | White powder |
| Formula | C21H24N2O4 | M.Wt | 368.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Ajmalicine oxindole B; Rubradinine | ||
| Solubility | Soluble in chloroform | ||
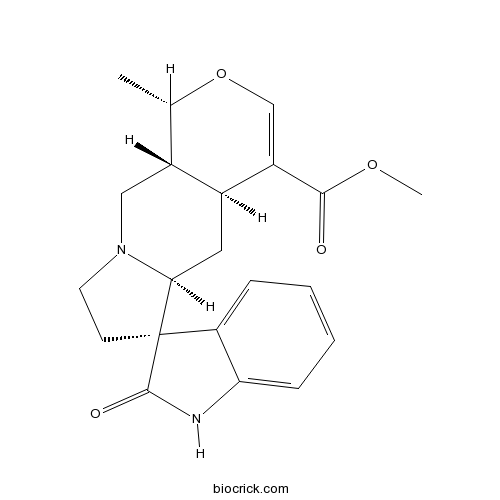
| Chemical Name | methyl (1S,4aS,5aS,6R,10aR)-1-methyl-2'-oxospiro[1,4a,5,5a,7,8,10,10a-octahydropyrano[3,4-f]indolizine-6,3'-1H-indole]-4-carboxylate | ||
| SMILES | CC1C2CN3CCC4(C3CC2C(=CO1)C(=O)OC)C5=CC=CC=C5NC4=O | ||
| Standard InChIKey | JMIAZDVHNCCPDM-DAFCLMLCSA-N | ||
| Standard InChI | InChI=1S/C21H24N2O4/c1-12-14-10-23-8-7-21(16-5-3-4-6-17(16)22-20(21)25)18(23)9-13(14)15(11-27-12)19(24)26-2/h3-6,11-14,18H,7-10H2,1-2H3,(H,22,25)/t12-,13-,14+,18-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Mitraphylline has antiproliferative effects on human glioma and neuroblastoma cell lines. 2. Mitraphylline has anti-inflammatory activity, it inhibits lipopolysaccharide-mediated activation of primary human neutrophils. |
| Targets | IL Receptor | TNF-α | NF-kB |

Mitraphylline Dilution Calculator

Mitraphylline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7144 mL | 13.5722 mL | 27.1444 mL | 54.2888 mL | 67.861 mL |
| 5 mM | 0.5429 mL | 2.7144 mL | 5.4289 mL | 10.8578 mL | 13.5722 mL |
| 10 mM | 0.2714 mL | 1.3572 mL | 2.7144 mL | 5.4289 mL | 6.7861 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5429 mL | 1.0858 mL | 1.3572 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5429 mL | 0.6786 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Strychnine phosphate
Catalog No.:BCC8257
CAS No.:509-42-2
- Napellonine
Catalog No.:BCN2536
CAS No.:509-24-0
- Aconine
Catalog No.:BCN2394
CAS No.:509-20-6
- Delsoline
Catalog No.:BCN5405
CAS No.:509-18-2
- Gelsemine
Catalog No.:BCN5804
CAS No.:509-15-9
- 1-Acetyl-beta-carboline
Catalog No.:BCN3101
CAS No.:50892-83-6
- WY-14643 (Pirinixic Acid)
Catalog No.:BCC2265
CAS No.:50892-23-4
- 1,4-Bis(2-benzoxazolyl)naphthalene
Catalog No.:BCC8423
CAS No.:5089-22-5
- Liensinine diperchlorate
Catalog No.:BCN6336
CAS No.:5088-90-4
- Neoliquiritin
Catalog No.:BCN6663
CAS No.:5088-75-5
- 1,5,6-Trihydroxy-3-methoxyxanthone
Catalog No.:BCN8121
CAS No.:50868-52-5
- Tetramisole HCl
Catalog No.:BCC4735
CAS No.:5086-74-8
- Arteannuin B
Catalog No.:BCN5623
CAS No.:50906-56-4
- Toxyloxanthone D
Catalog No.:BCN3070
CAS No.:50906-62-2
- Nemorensine
Catalog No.:BCN2099
CAS No.:50906-96-2
- Taiwanhomoflavone B
Catalog No.:BCN5624
CAS No.:509077-91-2
- 1beta-Hydroxytorilin
Catalog No.:BCN7095
CAS No.:509078-16-4
- IRAK-1-4 Inhibitor I
Catalog No.:BCC1659
CAS No.:509093-47-4
- 2-Amino-3,5-dibromobenzaldehyde
Catalog No.:BCC8523
CAS No.:50910-55-9
- Boc-D-Arg(NO2)-OH
Catalog No.:BCC2610
CAS No.:50913-12-7
- Bombinakinin M
Catalog No.:BCC5904
CAS No.:509151-65-9
- Mizoribine
Catalog No.:BCC4454
CAS No.:50924-49-7
- Verminoside
Catalog No.:BCN5625
CAS No.:50932-19-9
- Carminomycin
Catalog No.:BCC6379
CAS No.:50935-04-1, 39472-31-6
Aqueous extracts from Uncaria tomentosa (Willd. ex Schult.) DC. reduce bronchial hyperresponsiveness and inflammation in a murine model of asthma.[Pubmed:29432856]
J Ethnopharmacol. 2018 May 23;218:76-89.
ETHNOPHARMACOLOGICAL RELEVANCE: Uncaria tomentosa (Willd. Ex Schult) DC is used by indigenous tribes in the Amazonian region of Central and South America to treat inflammation, allergies and asthma. The therapeutic properties of U. tomentosa have been attributed to the presence of tetracyclic and pentacyclic oxindole alkaloids and to phenolic acids. AIMS OF THE STUDY: To characterize aqueous bark extracts (ABE) and aqueous leaf extracts (ALE) of U. tomentosa and to compare their anti-inflammatory effects. MATERIALS AND METHODS: Constituents of the extracts were identified by ultra performance liquid chromatography-mass spectrometry. Anti-inflammatory activities were assessed in vitro by exposing lipopolysaccharide-stimulated macrophage cells (RAW264.7-Luc) to ABE, ALE and standard Mitraphylline. In vivo assays were performed using a murine model of ovalbumin (OVA)-induced asthma. OVA-sensitized animals were treated with ABE or ALE while controls received dexamethasone or saline solution. Bronchial hyperresponsiveness, production of Th1 and Th2 cytokines, total and differential counts of inflammatory cells in the bronchoalveolar lavage (BAL) and lung tissue were determined. RESULTS: Mitraphylline, isoMitraphylline, chlorogenic acid and quinic acid were detected in both extracts, while isorhyncophylline and rutin were detected only in ALE. ABE, ALE and Mitraphylline inhibited the transcription of nuclear factor kappa-B in cell cultures, ALE and Mitraphylline reduced the production of interleukin (IL)-6, and Mitraphylline reduced production of tumor necrosis factor-alpha. Treatment with ABE and ALE at 50 and 200mgkg(-1), respectively, reduced respiratory elastance and tissue damping and elastance. ABE and ALE reduced the number of eosinophils in BAL, while ALE at 200mgkg(-1) reduced the levels of IL-4 and IL-5 in the lung homogenate. Peribronchial inflammation was significantly reduced by treatment with ABE and ALE at 50 and 100mgkg(-1) respectively. CONCLUSION: The results clarify for the first time the anti-inflammatory activity of U. tomentosa in a murine model of asthma. Although ABE and ALE exhibited distinct chemical compositions, both extracts inhibited the production of pro-inflammatory cytokines in vitro. In vivo assays revealed that ABE was more effective in treating asthmatic inflammation while ALE was more successful in controlling respiratory mechanics. Both extracts may have promising applications in the phytotherapy of allergic asthma.
Mitraphylline inhibits lipopolysaccharide-mediated activation of primary human neutrophils.[Pubmed:26926175]
Phytomedicine. 2016 Feb 15;23(2):141-8.
BACKGROUND: Mitraphylline (MTP) is the major pentacyclic oxindolic alkaloid presented in Uncaria tomentosa. It has traditionally been used to treat disorders including arthritis, heart disease, cancer, and other inflammatory diseases. However, the specific role of MTP is still not clear, with more comprehensivestudies, our understanding of this ancient herbal medicine will continue growing. HYPOTHESIS/PURPOSE: Some studies provided its ability to inhibit proinflamatory cytokines, such as TNF-alpha, through NF-kappaB-dependent mechanism. TNF-alpha primes neutrophils and modulates phagocytic and oxidative burst activities in inflammatory processes. Since, neutrophils represent the most abundant pool of leukocytes in human blood and play a crucial role in inflammation, we aimed to determine the ability of MTP to modulate neutrophil activation and differentially regulate inflammatory-related cytokines. METHODS: To determine the mechanism of action of MTP, we investigated the effects on LPS-activated human primary neutrophils responses including activation surface markers by FACS and the expression of inflammatory cytokines, measured by real time PCR and ELISA. RESULTS: Treatment with MTP reduced the LPS-dependent activation effects. Activated neutrophils (CD16(+)CD62L(-)) diminished after MTP administration. Moreover, proinflamatory cytokines (TNF-alpha, IL-6 or IL-8) expression and secretion were concomitantly reduced, similar to basal control conditions. CONCLUSION: Taken together, our results demonstrate that MTP is able to elicit an anti-inflammatory response that modulates neutrophil activation contributing to the attenuation of inflammatory episodes. Further studies are need to characterize the mechanism by which MTP can affect this pathway that could provide a means to develop MTP as new candidate for inflammatory disease therapies.
Pharmacological effects of mitraphylline from Uncaria tomentosa in primary human monocytes: Skew toward M2 macrophages.[Pubmed:25975515]
J Ethnopharmacol. 2015 Jul 21;170:128-35.
ETHNOPHARMACOLOGICAL RELEVANCE: Uncaria tomentosa (Willdenow ex Roemer & Schultes) DC. (Rubiaceae) is a Peruvian thorny liana, commonly known as "cats claw", and traditionally used in folk medicine to deal with several inflammatory diseases. Mitraphylline (MTP) is the most abundant pentacyclic oxindolic alkaloid (POA) from U. Tomentosa and has been reported to modify the inflammatory response. Herein, we have sought to identify the mechanisms underlying this modulatory effect of MTP on primary human monocytes and its ability to regulate differentiation processes on human primary monocyte and monocyte-derived macrophages. MATERIAL AND METHODS: In vitro studies with human primary monocytes and monocyte-derived macrophages were performed. Monocytes and M0 macrophages were exposed to MTP (25muM) and LPS (100ng/mL). M0 macrophages were polarized to M1 and M2 phenotypes in the absence or presence of MTP. The activation state of monocytes/macrophages was assessed by flow cytometry, gene expression and protein analysis of different specific markers. RESULTS: In human primary monocytes, the incubation of MTP for 24h reduced the number of classical (CD14(++)CD16(-)) and intermediate (CD14(++)CD16(+)) subsets when compared to untreated or LPS-treated cells. MTP also reduced the chemotactic capacity of human primary monocytes. In addition, MTP promoted the polarization of M0 macrophages toward an anti-inflammatory M2 phenotype, the abrogation of the release of pro-inflammatory cytokines such as TNFalpha, IL-6 or IL-1beta, as well as the restoration of markers for M2 macrophages in LPS-treated M1 macrophages. CONCLUSIONS: Our results suggest that MTP may be a key modulator for regulating the plasticity of monocytes/macrophages and the attenuation of the inflammatory response.
Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline.[Pubmed:24841968]
Planta Med. 2014 May;80(7):568-76.
Mitragyna speciosa (kratom) is a popular herb in Southeast Asia, which is traditionally used to treat withdrawal symptoms associated with opiate addiction. Mitragynine, 7-hydroxymitragynine, and Mitraphylline are reported to be the central nervous system active alkaloids which bind to the opiate receptors. Mitraphylline is also present in the bark of Uncaria tomentosa (cat's claw). Several therapeutic properties have been reported for these compounds but limited information is available on the absorption and distribution properties. This study focuses on evaluating the absorption, distribution, metabolism, and excretion (ADME) properties of these compounds and their effect on major efflux transporter P-glycoprotein, using in vitro methods. Quantitative analysis was performed by the Q-TOF LC-MS system. Mitragynine was unstable in simulated gastric fluid with 26 % degradation but stable in simulated intestinal fluid. 7-Hydroxymitragynine degraded up to 27 % in simulated gastric fluid, which could account for its conversion to mitragynine (23 %), while only 6 % degradation was seen in simulated intestinal fluid. Mitraphylline was stable in simulated gastric fluid but unstable in simulated intestinal fluid (13.6 % degradation). Mitragynine and 7-hydroxymitragynine showed moderate permeability across Caco-2 and MDR-MDCK monolayers with no significant efflux. However, Mitraphylline was subjected to efflux mediated by P-glycoprotein in both Caco-2 and MDR-MDCK monolayers. Mitragynine was found to be metabolically stable in both human liver microsomes and S9 fractions. In contrast, both 7-hydroxymitragynine and Mitraphylline were metabolized by human liver microsomes with half-lives of 24 and 50 min, respectively. All three compounds exhibited high plasma protein binding (> 90 %) determined by equilibrium dialysis. Mitragynine and 7-hydroxymitragynine inhibited P-glycoprotein with EC50 values of 18.2 +/- 3.6 microM and 32.4 +/- 1.9 microM, respectively, determined by the calcein-AM fluorescent assay, while no inhibition was seen with Mitraphylline. These data indicate the possibility of a drug interaction if mitragynine and 7-hydroxymitragynine are coadministered with drugs that are P-glycoprotein substrates.
Cat's claw oxindole alkaloid isomerization induced by cell incubation and cytotoxic activity against T24 and RT4 human bladder cancer cell lines.[Pubmed:23975868]
Planta Med. 2013 Oct;79(15):1413-20.
The antitumor activity of Uncaria tomentosa, a native vine from the Amazonian rainforest, has been ascribed to pentacyclic oxindole alkaloids occurring in its bark. Former studies have shown that this activity, as well as its intensity, depends on whether cat's claw alkaloids occur as original compounds or isomerized derivatives. This work addresses this aspect, using T24 and RT4 human bladder cancer cell lines for that purpose. Bark samples were extracted by dynamic maceration, prepurified with cross-linked polyvinylpyrrolidone and properly fractioned by an ion exchange process to obtain an oxindole alkaloid purified fraction. Alkaloid isomerization was induced by heating it under reflux at 85 degrees C. Samples collected after 5, 15, and 45 min of heating were analyzed by HPLC-PDA, freeze-dried at once, and separately assayed using the non-isomerized purified fraction for comparison purposes. The latter showed significant and dose-dependent cytotoxic activity against both T24 and RT4 cancer cell lines (IC50: 164.13 and 137.23 microg/mL, respectively). However, results for both cell lines were equivalent to those observed for isomerized samples (p > 0.05). The alkaloid isomerization induced by the incubation conditions (buffered medium pH 7.4 and temperature 37 degrees C) helps to explain the similar results obtained from non-isomerized and isomerized samples. Mitraphylline, speciophylline, uncarine F, and, to a lesser degree, pteropodine were more susceptible to isomerization under the incubation conditions. Thus, the alkaloid profile of all fractions and their cytotoxic activities against T24 and RT4 human bladder cancer cell lines are determined to a large extent by the incubation conditions.


