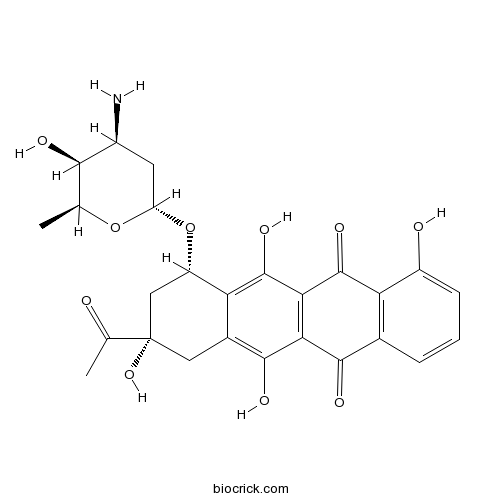
Carminomycinantitumor antibiotic CAS# 50935-04-1, 39472-31-6 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- L-Stepholidine
Catalog No.:BCN2599
CAS No.:16562-13-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 50935-04-1, 39472-31-6 | SDF | Download SDF |
| PubChem ID | 443831 | Appearance | Powder |
| Formula | C26H27NO10 | M.Wt | 513.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7H-tetracene-5,12-dione | ||
| SMILES | CC1C(C(CC(O1)OC2CC(CC3=C(C4=C(C(=C23)O)C(=O)C5=C(C4=O)C=CC=C5O)O)(C(=O)C)O)N)O | ||
| Standard InChIKey | XREUEWVEMYWFFA-CSKJXFQVSA-N | ||
| Standard InChI | InChI=1S/C26H27NO10/c1-9-21(30)13(27)6-16(36-9)37-15-8-26(35,10(2)28)7-12-18(15)25(34)20-19(23(12)32)22(31)11-4-3-5-14(29)17(11)24(20)33/h3-5,9,13,15-16,21,29-30,32,34-35H,6-8,27H2,1-2H3/t9-,13-,15-,16-,21+,26-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Carminomycin Dilution Calculator

Carminomycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9475 mL | 9.7373 mL | 19.4746 mL | 38.9492 mL | 48.6864 mL |
| 5 mM | 0.3895 mL | 1.9475 mL | 3.8949 mL | 7.7898 mL | 9.7373 mL |
| 10 mM | 0.1947 mL | 0.9737 mL | 1.9475 mL | 3.8949 mL | 4.8686 mL |
| 50 mM | 0.0389 mL | 0.1947 mL | 0.3895 mL | 0.779 mL | 0.9737 mL |
| 100 mM | 0.0195 mL | 0.0974 mL | 0.1947 mL | 0.3895 mL | 0.4869 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Carminomycin is a new antitumor antibiotic [1].
Antibiotics are a type of antimicrobial used in the treatment of bacterial infection. They can inhibit the growth of bacteria. Antitumor antibiotics are effective agents widely used in cancer chemotherapy [2].
Carminomycin is a new antitumor antibiotic isolated from the mycelium of Actinomadura carminata containing seven components, five of which are biologically active. The more interesting components are components 1, 2 and 3 [1]. In human MCF-7 breast carcinoma and human K562 leukemia cell lines, carminomycin inhibited cell growth with IC50 values of 90 and 60 nM, respectively. In Pgp-expressing MCF-7Dox and K562i/S9 cell lines, carminomycin inhibited cell growth with a similar activity compared with wild type cells, which suggested that carminomycin could circumvent Pgp-mediated multidrug resistant (MDR) [2]. In Micrococcus luteus cells, carminomycin induced one-thread breaks in DNA. In mutant strain DB-7 of M. luteus, carminomycin was more difficult to induce the one-thread breaks, suggesting that UV-endonuclease was probably involved in reparation of the DNA damages induced by carminomycin [3].
In DBA/2 mice with leukemia L-1210, carminomycin (1.5 mg/kg) inhibited the lymphoma colonies by 50% [4].
References:
[1]. Brazhnikova MG, Zbarsky VB, Ponomarenko VI, et al. Physical and chemical characteristics and structure of carminomycin, a new antitumor antibiotic. J Antibiot (Tokyo), 1974, 27(4): 254-259.
[2]. Tevyashova AN, Shtil AA, Olsufyeva EN, et al. Carminomycin, 14-hydroxycarminomycin and its novel carbohydrate derivatives potently kill human tumor cells and their multidrug resistant variants. J Antibiot (Tokyo), 2004, 57(2): 143-150.
[3]. Trenin AS. Carminomycin induction of single-stranded DNA breaks in Micrococcus luteus cells. Antibiotiki, 1979, 24(11): 841-846.
[4]. Berezina TA, Uteshev BS. Comparative characteristics of the antitumor and immunodepressive activity of carminomycin on the L-1210 experimental model. Antibiotiki, 1979, 24(10): 767-771.
- Verminoside
Catalog No.:BCN5625
CAS No.:50932-19-9
- Mizoribine
Catalog No.:BCC4454
CAS No.:50924-49-7
- Bombinakinin M
Catalog No.:BCC5904
CAS No.:509151-65-9
- Boc-D-Arg(NO2)-OH
Catalog No.:BCC2610
CAS No.:50913-12-7
- 2-Amino-3,5-dibromobenzaldehyde
Catalog No.:BCC8523
CAS No.:50910-55-9
- IRAK-1-4 Inhibitor I
Catalog No.:BCC1659
CAS No.:509093-47-4
- 1beta-Hydroxytorilin
Catalog No.:BCN7095
CAS No.:509078-16-4
- Taiwanhomoflavone B
Catalog No.:BCN5624
CAS No.:509077-91-2
- Nemorensine
Catalog No.:BCN2099
CAS No.:50906-96-2
- Toxyloxanthone D
Catalog No.:BCN3070
CAS No.:50906-62-2
- Arteannuin B
Catalog No.:BCN5623
CAS No.:50906-56-4
- Mitraphylline
Catalog No.:BCC8213
CAS No.:509-80-8
- Anacrotine
Catalog No.:BCN2057
CAS No.:5096-49-1
- Crotanecine
Catalog No.:BCN1963
CAS No.:5096-50-4
- Canadine
Catalog No.:BCN5626
CAS No.:5096-57-1
- N-Methylcoclaurine
Catalog No.:BCN7079
CAS No.:5096-70-8
- 16-Methoxystrychnidin-10-One
Catalog No.:BCN8472
CAS No.:5096-72-0
- 7ACC1
Catalog No.:BCC5553
CAS No.:50995-74-9
- Pronethalol hydrochloride
Catalog No.:BCC5678
CAS No.:51-02-5
- Procaine HCl
Catalog No.:BCC5072
CAS No.:51-05-8
- Benzimidazole
Catalog No.:BCC8847
CAS No.:51-17-2
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Tiratricol
Catalog No.:BCC4738
CAS No.:51-24-1
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
Anthraquinone-induced cell injury: acute toxicity of carminomycin, epirubicin, idarubicin and mitoxantrone in isolated cardiomyocytes.[Pubmed:10454220]
Toxicology. 1999 Jul 1;135(1):11-20.
Acute toxic effects of the antineoplastic anthraquinones Carminomycin, epirubicin, idarubicin and mitoxantrone were studied in primary cultures of cardiomyocytes, which were isolated from adult rats. Both time- and concentration-dependent changes of cell structure and viability (trypan blue exclusion) following incubation of myocytes with subclinical, clinical and toxic concentrations of the anthraquinones were examined by light microscopy. The area under the decay curve of viable and rod-shaped myocytes was used to express cytotoxicity of the drugs. Mitoxantrone was found to reduce cell viability and number of rod-shaped cells to the greatest extent, followed by Carminomycin, idarubicin and epirubicin. A significantly lower accumulation in cardiomyocytes was obtained with epirubicin and idarubicin compared with Carminomycin. An inhibitory effect on oxygen consumption by the cells occurred already at 0.1 microM with epirubicin, whereas inhibition caused by other anthraquinones was less pronounced. Our data indicate a weak association of net accumulation and the toxicity parameter IC50 for Carminomycin and idarubicin. In contrast to these results, a more significant correlation of cytotoxicity and anthraquinone lipophilicity was found, which suggests that the lipophilic character of a particular anthraquinone may be an important factor in drug-induced acute cardiotoxicity.
Carminomycin, 14-hydroxycarminomycin and its novel carbohydrate derivatives potently kill human tumor cells and their multidrug resistant variants.[Pubmed:15112963]
J Antibiot (Tokyo). 2004 Feb;57(2):143-50.
The new hydrophilic derivatives of 14-hydroxyCarminomycin were obtained using 13-dimethyl ketal of 14-bromoCarminomycin (6) as the starting compound. The reductive alkylation of 6 with melibiose or D-galactose followed by hydrolysis of the corresponding intermediate bromoketals 9 and 11 produced 3'-N-[-alpha-D-(galactopyranosyl-(1 --> 6)-O-D-1-desoxyglucit-1-yl]-14-hydroxyCarminomycin (10) and 3'-N-(1-desoxy-D-galactit-1-yl)-14-hydroxyCarminomycin (12), respectively. These novel derivatives 10 and 12 were less toxic than Carminomycin or 14-hydroxyCarminomycin for leukemia (K562) and breast carcinoma (MCF-7) cells. Importantly, Carminomycin, 14-hydroxyCarminomycin and compounds 10 and 12 were similarly active for wild type cells and their multidrug resistant (MDR) sublines, K562i/S9 and MCF-7Dox.
[Optimization of conditions of preparative chromatography of carminomycin on a carboxylic cation exchanger].[Pubmed:11962206]
Prikl Biokhim Mikrobiol. 2002 Mar-Apr;38(2):128-31.
Time course of equilibrium and nonequilibrium sorption of Carminomycin on carboxylic cation exchanger BDM-12 has been studied. Physicochemical requirements and limits of mobile phase flow rate are determined for the regular mode of preparative chromatography under the conditions of sharpening of the chromatographic zone.
Carminomycin I is an apoptosis inducer that targets the Golgi complex in clear cell renal carcinoma cells.[Pubmed:21199801]
Cancer Res. 2011 Jan 1;71(1):134-42.
Clear cell renal cell carcinoma (CCRCC) evolves due to mutations in the Von Hippel-Lindau (VHL) tumor suppressor gene. Although the loss of VHL enables survival and proliferation of CCRCC cells, it is also expected to introduce vulnerabilities that may be exploited for therapeutics discovery. To this end, we developed a high-throughput screen to identify small molecules derived from plants, microorganisms, and marine organisms to which CCRCC cells are sensitive. Screening over 8,000 compounds using this approach, we report here the identification of the microbially derived compound Carminomycin I (CA) as an effective inhibitor of VHL-defective (VHL(-/-)) CCRCC cell proliferation. CA also induced apoptosis in CCRCC cells by a mechanism independent of p53 or hypoxia-inducible factor 2. We found that P-glycoprotein (P-gp) sequestered CA within the Golgi complex. Interestingly, Golgi sequestration was critical for the antiproliferative effects of CA and P-gp inhibitors abrogated this activity. Furthermore, CA induced cleavage of the Golgi protein p115 and the translocation of its C-terminal fragment to the nucleus. Finally, examination of the activity of the VHL-interacting Golgi protein, endoplasmic reticulum-Golgi intermediate compartment, ERGIC-53 showed that VHL could mediate protection from CA in CCRCC cells. Our natural product-based screening approach has revealed the P-gp-mediated localization of anticancer compounds within the Golgi in CCRCC cells as a potential strategy of targeting VHL-deficient CCRCC cells.


