Uncarine FCAS# 14019-66-0 |
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
- Mitraphylline
Catalog No.:BCC8213
CAS No.:509-80-8
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Uncarine A
Catalog No.:BCN7767
CAS No.:6899-73-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 14019-66-0 | SDF | Download SDF |
| PubChem ID | 12304288 | Appearance | Powder |
| Formula | C21H24N2O4 | M.Wt | 368.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
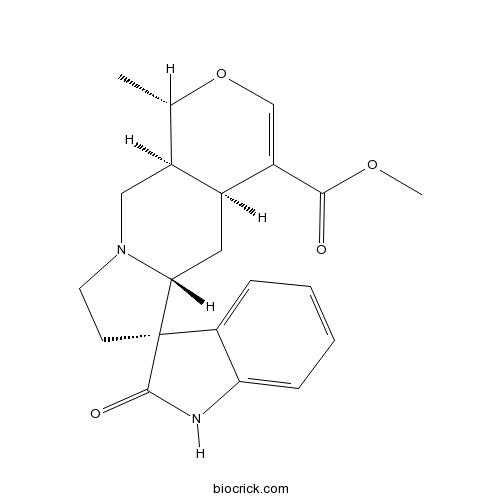
| Chemical Name | methyl (1S,4aS,5aR,6R,10aS)-1-methyl-2'-oxospiro[1,4a,5,5a,7,8,10,10a-octahydropyrano[3,4-f]indolizine-6,3'-1H-indole]-4-carboxylate | ||
| SMILES | CC1C2CN3CCC4(C3CC2C(=CO1)C(=O)OC)C5=CC=CC=C5NC4=O | ||
| Standard InChIKey | JMIAZDVHNCCPDM-PMJXBNNDSA-N | ||
| Standard InChI | InChI=1S/C21H24N2O4/c1-12-14-10-23-8-7-21(16-5-3-4-6-17(16)22-20(21)25)18(23)9-13(14)15(11-27-12)19(24)26-2/h3-6,11-14,18H,7-10H2,1-2H3,(H,22,25)/t12-,13-,14-,18+,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Uncarine F Dilution Calculator

Uncarine F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7144 mL | 13.5722 mL | 27.1444 mL | 54.2888 mL | 67.861 mL |
| 5 mM | 0.5429 mL | 2.7144 mL | 5.4289 mL | 10.8578 mL | 13.5722 mL |
| 10 mM | 0.2714 mL | 1.3572 mL | 2.7144 mL | 5.4289 mL | 6.7861 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5429 mL | 1.0858 mL | 1.3572 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5429 mL | 0.6786 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Angustine
Catalog No.:BCN9445
CAS No.:40041-96-1
- Caloxanthone B
Catalog No.:BCN9444
CAS No.:155233-17-3
- Stipuleanoside R1
Catalog No.:BCN9443
CAS No.:96627-79-1
- Adenostemmoic acid C
Catalog No.:BCN9442
CAS No.:130217-18-4
- Withaphysalin C
Catalog No.:BCN9441
CAS No.:57485-60-6
- ent-Toddalolactone
Catalog No.:BCN9440
CAS No.:1570054-19-1
- 1-Oxohederagenin
Catalog No.:BCN9439
CAS No.:618390-67-3
- 1,5-Epoxy-3-hydroxy-1-(3,4-dihydroxy-5-methoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane
Catalog No.:BCN9438
CAS No.:182227-93-6
- Cucurbitacin IIa 2-O-glucoside
Catalog No.:BCN9437
CAS No.:77704-34-8
- [4]-Gingerdiol
Catalog No.:BCN9436
CAS No.:53254-75-4
- Physaminimin N
Catalog No.:BCN9435
CAS No.:2131235-87-3
- Orthosiphol B
Catalog No.:BCN9434
CAS No.:144078-08-0
- ent-15α-Acetoxy-11α-hydroxykaur-16-en-19-oic acid
Catalog No.:BCN9447
CAS No.:70324-38-8
- (-)-Toddalolactone 3′-O-β-D-glucopyranoside
Catalog No.:BCN9448
CAS No.:1176645-57-0
- 12-O-Methylinophyllum A
Catalog No.:BCN9449
CAS No.:2131757-10-1
- Seselin
Catalog No.:BCN9450
CAS No.:523-59-1
- 5-Methoxyseselin
Catalog No.:BCN9451
CAS No.:31525-76-5
- 3,6-Dihydroxy-1,2,7-trimethoxyxanthone
Catalog No.:BCN9452
CAS No.:916210-79-2
- Trigochinin A
Catalog No.:BCN9453
CAS No.:1210299-29-8
- Isozedoarondiol
Catalog No.:BCN9454
CAS No.:108887-68-9
- 1,8-Dihydroxy-p-menth-3-en-2-one
Catalog No.:BCN9455
CAS No.:1392224-56-4
- Toddalolactone 3′-O-ethyl ether
Catalog No.:BCN9456
CAS No.:1538607-30-5
- Schisandrolic acid
Catalog No.:BCN9457
CAS No.:55511-17-6
- Toddalosin ethyl ether
Catalog No.:BCN9458
CAS No.:1538607-31-6
Cat's claw oxindole alkaloid isomerization induced by cell incubation and cytotoxic activity against T24 and RT4 human bladder cancer cell lines.[Pubmed:23975868]
Planta Med. 2013 Oct;79(15):1413-20.
The antitumor activity of Uncaria tomentosa, a native vine from the Amazonian rainforest, has been ascribed to pentacyclic oxindole alkaloids occurring in its bark. Former studies have shown that this activity, as well as its intensity, depends on whether cat's claw alkaloids occur as original compounds or isomerized derivatives. This work addresses this aspect, using T24 and RT4 human bladder cancer cell lines for that purpose. Bark samples were extracted by dynamic maceration, prepurified with cross-linked polyvinylpyrrolidone and properly fractioned by an ion exchange process to obtain an oxindole alkaloid purified fraction. Alkaloid isomerization was induced by heating it under reflux at 85 degrees C. Samples collected after 5, 15, and 45 min of heating were analyzed by HPLC-PDA, freeze-dried at once, and separately assayed using the non-isomerized purified fraction for comparison purposes. The latter showed significant and dose-dependent cytotoxic activity against both T24 and RT4 cancer cell lines (IC50: 164.13 and 137.23 microg/mL, respectively). However, results for both cell lines were equivalent to those observed for isomerized samples (p > 0.05). The alkaloid isomerization induced by the incubation conditions (buffered medium pH 7.4 and temperature 37 degrees C) helps to explain the similar results obtained from non-isomerized and isomerized samples. Mitraphylline, speciophylline, Uncarine F, and, to a lesser degree, pteropodine were more susceptible to isomerization under the incubation conditions. Thus, the alkaloid profile of all fractions and their cytotoxic activities against T24 and RT4 human bladder cancer cell lines are determined to a large extent by the incubation conditions.
Anxiogenic-like effects of Uncaria tomentosa (Willd.) DC. aqueous extract in an elevated plus maze test in mice: a preliminary study.[Pubmed:23327494]
Nat Prod Res. 2013;27(18):1682-5.
The purpose of this study was to examine the effect of orally administered Uncaria tomentosa aqueous extracts (UTE) (Willd. ex Roem. & Schult.) DC. (Rubiaceae) during 7, 15, 30 and 90 days of treatment on the expression of anxiety, as expressed in the elevated plus maze test in male Albino Swiss mice. UTE revealed an anxiogenic effect in relation to the control group at 15 and 30 days, but it was reversed after 90 days of administration, without affecting the locomotor activity or any deleterious effects on the overall performance of the animal, either for its ambulation, or clinical status, and body weight and organ weight/body weight from liver, lung and kidney were unaffected. These biphasic effects are usually indicative of heterogeneity in sites of action due to the presence of many alkaloids (speciophylline, Uncarine F and uncarine E) and flavanols (catechin and epigallocatechin) identified and isolated from UTE.
Differential alkaloid profile in Uncaria tomentosa micropropagated plantlets and root cultures.[Pubmed:23296316]
Biotechnol Lett. 2013 May;35(5):791-7.
The alkaloids of Uncaria tomentosa micropropagated plantlets and root cultures were isolated and identified by NMR and mass spectrometry. Plantlets yielded pteropodine (1), isopteropodine (2), mitraphylline (3), isomitraphylline (4), Uncarine F (5), speciophylline (6), rhynchophylline (7) and isorhynchophylline (8). In plantlets growing under continuous light, tetracyclic alkaloids 7 and 8 decreased from 20 +/- 1.8 at 2 months to 2.2 +/- 0.33 mg/g dry wt at 6 months, while the pentacyclic alkaloids 1-4 increased from 7.7 +/- 1.4 to 15 +/- 0.05 mg/g dry wt, supporting their biogenetic conversion. Micropropagated plantlets produced four times more alkaloids (27.6 +/- 3.1 mg/g dry wt) than greenhouse plants. Plantlet roots yielded 3, 4, 8 and the glucoindole alkaloids 3alpha-dihydrocadambine (9) and dolichantoside (10), the last one not previously found in Uncaria.
A new monoterpenoid oxindole alkaloid from Hamelia patens micropropagated plantlets.[Pubmed:23285803]
Nat Prod Commun. 2012 Nov;7(11):1441-4.
Chemical studies on Hamelia patens (Rubiaceae) micropropagated plantlets allowed production of a new monoterpenoid oxindole alkaloid, named (-)-hameline (7), together with eight known alkaloids, tetrahydroalstonine (1), aricine (2), pteropodine (3), isopteropodine (4), Uncarine F (5), speciophylline (6), palmirine (8), and rumberine (9). The structure of the new alkaloid was assigned on the basis of 1D and 2D NMR spectroscopy, mass spectrometry, and molecular modeling.
Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemia cells.[Pubmed:16445836]
Br J Haematol. 2006 Mar;132(5):615-22.
Natural products are still an untapped source of promising lead compounds for the generation of antineoplastic drugs. Here, we investigated for the first time the antiproliferative and apoptotic effects of highly purified oxindole alkaloids, namely isopteropodine (A1), pteropodine (A2), isomitraphylline (A3), Uncarine F (A4) and mitraphylline (A5) obtained from Uncaria tomentosa, a South American Rubiaceae, on human lymphoblastic leukaemia T cells (CCRF-CEM-C7H2). Four of the five tested alkaloids inhibited proliferation of acute lymphoblastic leukaemia cells. Furthermore, the antiproliferative effect of the most potent alkaloids pteropodine (A2) and Uncarine F (A4) correlated with induction of apoptosis. After 48 h, 100 micromol/l A2 or A4 increased apoptotic cells by 57%. CEM-C7H2 sublines with tetracycline-regulated expression of bcl-2, p16ink4A or constitutively expressing the cowpox virus protein crm-A were used for further studies of the apoptosis-inducing properties of these alkaloids. Neither overexpression of bcl-2 or crm-A nor cell-cycle arrest in G0/G1 phase by tetracycline-regulated expression of p16INK4A could prevent alkaloid-induced apoptosis. Our results show the strong apoptotic effects of pteropodine and Uncarine F on acute leukaemic lymphoblasts and recommend the alkaloids for further studies in xenograft models.
Alkaloids and procyanidins of an Uncaria sp. from Peru.[Pubmed:947783]
Farmaco Sci. 1976 Jul;31(7):527-35.
The alkaloid and procyanidin composition of Uncaria sp. from eastern Peru, used in folk medicine was studied. Five alkaloids have been separated and identified as pteropodine, speciophylline, isopteropodine, Uncarine F and isomytraphylline, all belonging to the oxindole group characteristic of the Rubiaceae. Moreover (--) epicatechin and four dimeric procyanidins A1, B1, B2 and B4 have been shown to constitute the polyphenolic fraction of the plant extract.


