Uncarine CCAS# 5629-60-7 |
- Uncarine D
Catalog No.:BCC8262
CAS No.:4697-68-1
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
- Mitraphylline
Catalog No.:BCC8213
CAS No.:509-80-8
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Uncarine A
Catalog No.:BCN7767
CAS No.:6899-73-6
- Uncarine F
Catalog No.:BCN9446
CAS No.:14019-66-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 5629-60-7 | SDF | Download SDF |
| PubChem ID | 10429112 | Appearance | White powder |
| Formula | C21H24N2O4 | M.Wt | 368.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Pteropodine | ||
| Solubility | Soluble in chloroform and methan | ||
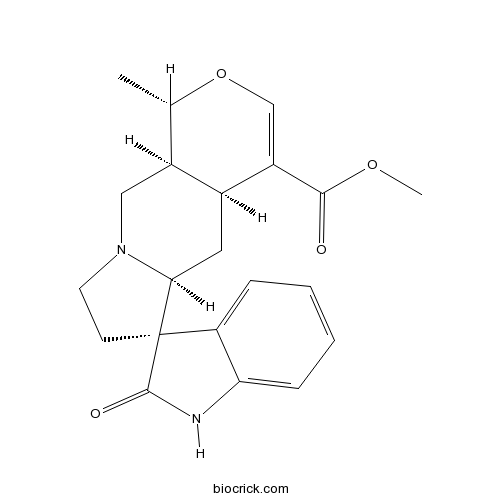
| Chemical Name | methyl (1S,4aS,5aS,6R,10aS)-1-methyl-2'-oxospiro[1,4a,5,5a,7,8,10,10a-octahydropyrano[3,4-f]indolizine-6,3'-1H-indole]-4-carboxylate | ||
| SMILES | CC1C2CN3CCC4(C3CC2C(=CO1)C(=O)OC)C5=CC=CC=C5NC4=O | ||
| Standard InChIKey | JMIAZDVHNCCPDM-QLMFUGSGSA-N | ||
| Standard InChI | InChI=1S/C21H24N2O4/c1-12-14-10-23-8-7-21(16-5-3-4-6-17(16)22-20(21)25)18(23)9-13(14)15(11-27-12)19(24)26-2/h3-6,11-14,18H,7-10H2,1-2H3,(H,22,25)/t12-,13-,14-,18-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pteropodine acts as a positive modulator of muscarinic M(1) and 5-HT(2) receptors. 2. Pteropodine shows antigenotoxic, antioxidant and lymphocyte induction effects. 3. Pteropodine shows strong apoptotic effect on acute leukaemic lymphoblasts. |
| Targets | Calcium Channel | 5-HT Receptor | Bcl-2/Bax |

Uncarine C Dilution Calculator

Uncarine C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7144 mL | 13.5722 mL | 27.1444 mL | 54.2888 mL | 67.861 mL |
| 5 mM | 0.5429 mL | 2.7144 mL | 5.4289 mL | 10.8578 mL | 13.5722 mL |
| 10 mM | 0.2714 mL | 1.3572 mL | 2.7144 mL | 5.4289 mL | 6.7861 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5429 mL | 1.0858 mL | 1.3572 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5429 mL | 0.6786 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anigorufone
Catalog No.:BCN7171
CAS No.:56252-32-5
- 2-O-Methylanigorufone
Catalog No.:BCN7180
CAS No.:56252-05-2
- Hydroxyanigorufone
Catalog No.:BCN7184
CAS No.:56252-02-9
- Cyclo(Leu-Val)
Catalog No.:BCN2436
CAS No.:5625-50-3
- Cyclo(Tyr-Gly)
Catalog No.:BCN2414
CAS No.:5625-49-0
- Porson
Catalog No.:BCN5750
CAS No.:56222-03-8
- Methyl lycernuate A
Catalog No.:BCN5749
CAS No.:56218-46-3
- Torsemide
Catalog No.:BCC4871
CAS No.:56211-40-6
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
- CGP 13501
Catalog No.:BCC7097
CAS No.:56189-68-5
- Acarbose
Catalog No.:BCC1190
CAS No.:56180-94-0
- H-Sar-OtBu.HCl
Catalog No.:BCC3336
CAS No.:5616-81-9
- Aloperine
Catalog No.:BCN8466
CAS No.:56293-29-9
- Fluoxetine HCl
Catalog No.:BCC1191
CAS No.:56296-78-7
- 3-(2,4-Dihydroxyphenyl)propionic acid
Catalog No.:BCN5751
CAS No.:5631-68-5
- Trimethylapigenin
Catalog No.:BCN8081
CAS No.:5631-70-9
- Kaempferol 3-O-(6''-galloyl)-beta-D-glucopyranoside
Catalog No.:BCN1416
CAS No.:56317-05-6
- 5,6,7,8-Tetramethoxycoumarin
Catalog No.:BCN5752
CAS No.:56317-15-8
- Moracin M
Catalog No.:BCN3292
CAS No.:56317-21-6
- Conocarpan acetate
Catalog No.:BCN7584
CAS No.:56319-04-1
- Hedychenone
Catalog No.:BCN5753
CAS No.:56324-54-0
- Oxybutynin
Catalog No.:BCC3833
CAS No.:5633-20-5
- Capillarisin
Catalog No.:BCN2461
CAS No.:56365-38-9
- Tirotundin
Catalog No.:BCN5754
CAS No.:56377-67-4
Preparative separation of six rhynchophylla alkaloids from Uncaria macrophylla wall by pH-zone refining counter-current chromatography.[Pubmed:24352009]
Molecules. 2013 Dec 12;18(12):15490-500.
pH-Zone refining counter-current chromatography was successfully applied to the preparative isolation and purification of six alkaloids from the ethanol extracts of Uncaria macrophylla Wall. Because of the low content of alkaloids (about 0.2%, w/w) in U. macrophylla Wall, the target compounds were enriched by pH-zone refining counter-current chromatography using a two-phase solvent system composed of petroleum ether-ethyl acetate-isopropanol-water (2:6:3:9, v/v), adding 10 mM triethylamine in organic stationary phase and 5 mM hydrochloric acid in aqueous mobile phase. Then pH-zone refining counter-current chromatography using the other two-phase solvent system was used for final purification. Six target compounds were finally isolated and purified by following two-phase solvent system composed of methyl tert-butyl ether (MTBE)-acetonitrile-water (4:0.5:5, v/v), adding triethylamine (TEA) (10 mM) to the organic phase and HCl (5 mM) to aqueous mobile phase. The separation of 2.8 g enriched total alkaloids yielded 36 mg hirsutine, 48 mg hirsuteine, 82 mg Uncarine C, 73 mg uncarine E, 163 mg rhynchophylline, and 149 mg corynoxeine, all with purities above 96% as verified by HPLC Their structures were identified by electrospray ionization-mass spectrometry (ESI-MS) and 1H-NMR spectroscopy.
(13)C, (15)N CPMAS NMR and GIAO DFT calculations of stereoisomeric oxindole alkaloids from Cat's Claw (Uncaria tomentosa).[Pubmed:19019638]
Solid State Nucl Magn Reson. 2008 Nov;34(4):202-9.
Oxindole alkaloids, isolated from the bark of Uncaria tomentosa [Willd. ex Schult.] Rubiaceae, are considered to be responsible for the biological activity of this herb. Five pentacyclic and two tetracyclic alkaloids were studied by solid-state NMR and theoretical GIAO DFT methods. The (13)C and (15)N CPMAS NMR spectra were recorded for mitraphylline, isomitraphylline, pteropodine (Uncarine C), isopteropodine (uncarine E), speciophylline (uncarine D), rhynchophylline and isorhynchophylline. Theoretical GIAO DFT calculations of shielding constants provide arguments for identification of asymmetric centers and proper assignment of NMR spectra. These alkaloids are 7R/7S and 20R/20S stereoisomeric pairs. Based on the (13)C CP MAS chemical shifts the 7S alkaloids (delta C3 70-71ppm) can be easily and conveniently distinguished from 7R (deltaC3 74.5-74.9ppm), also 20R (deltaC20 41.3-41.7ppm) from the 20S (deltaC20 36.3-38.3ppm). The epiallo-type isomer (3R, 20S) of speciophylline is characterized by a larger (15)N MAS chemical shift of N4 (64.6ppm) than the allo-type (3S, 20S) of isopteropodine (deltaN4 53.3ppm). (15)N MAS chemical shifts of N1-H in pentacyclic alkaloids are within 131.9-140.4ppm.
Investigation of Una De Gato I. 7-Deoxyloganic acid and 15N NMR spectroscopic studies on pentacyclic oxindole alkaloids from Uncaria tomentosa.[Pubmed:11397448]
Phytochemistry. 2001 Jul;57(5):781-5.
The C-8-(S) isomer of deoxyloganic acid (7-deoxyloganic acid), together with beta-sitosteryl glucoside, five known stereoisomeric pentacyclic oxindole alkaloids and the tetracyclic oxindole isorhyncophylline, were isolated from the inner bark of Uncaria tomentosa. Structures of the isolated compounds were based on 1H and 13C NMR data, mainly 2D NMR experiments, including 1H-13C HMBC and 1H-1H NOESY correlation. Furthermore, the hitherto unreported 15N chemical shifts of the isomeric oxindole alkaloids, using 1H-15N HMBC experiments, were utilized to facilitate their characterization. Uncarine D showed weak cytotoxic activity against SK-MEL, KB, BT-549 and SK-OV-3 cell lines with IC(50) values between 30 and 40 microg/ml, while Uncarine C exhibited weak cytotoxicity only against ovarian carcinoma (IC(50) at 37 microg/ml).
Two stereoisomeric pentacyclic oxindole alkaloids from Uncaria tomentosa: uncarine C and uncarine E.[Pubmed:11313600]
Acta Crystallogr C. 2001 Apr;57(Pt 4):480-2.
The chloroform solvate of Uncarine C (pteropodine), (1'S,3R,4'aS,5'aS,10'aS)-1,2,5',5'a,7',8',10',10'a-octahydro-1'-methyl-2-oxospiro [3H-indole-3,6'(4'aH)-[1H]pyrano[3,4-f]indolizine]-4'-carboxylic acid methyl ester, C(21)H(24)N(2)O(4).CHCl(3), has an absolute configuration with the spiro C atom in the R configuration. Its epimer at the spiro C atom, uncarine E (isopteropodine), (1'S,3S,4'aS,5'aS,10'aS)-1,2,5',5'a,7',8',10',10'a-octahydro-1'-methyl-2-oxospiro [3H-indole-3,6'(4'aH)-[1H]pyrano[3,4-f]indolizine]-4'-carboxylic acid methyl ester, C(21)H(24)N(2)O(4), has Z' = 3, with no solvent. Both form intermolecular hydrogen bonds involving only the oxindole, with N.O distances in the range 2.759 (4)-2.894 (5) A.
Effects of Uncaria tomentosa total alkaloid and its components on experimental amnesia in mice: elucidation using the passive avoidance test.[Pubmed:11197086]
J Pharm Pharmacol. 2000 Dec;52(12):1553-61.
The effects of Uncaria tomentosa total alkaloid and its oxindole alkaloid components, uncarine E, Uncarine C, mitraphylline, rhynchophylline and isorhynchophylline, on the impairment of retention performance caused by amnesic drugs were investigated using a step-down-type passive avoidance test in mice. In this test, the retention performance of animals treated with the amnesic and test drugs before training was assessed 24 h after training. Uncaria tomentosa total alkaloid (10-20 mg kg(-1), i.p.) and the alkaloid components (10-40 mg kg(-1), i.p.), as well as the muscarinic receptor agonist oxotremorine (0.01 mg kg(-1), i.p.), significantly attenuated the deficit in retention performance induced by the muscarinic receptor antagonist scopolamine (3 mg kg(-1), i.p.). The effective doses of Uncarine C and mitraphylline were larger than those of other alkaloid components. Uncarine E (20 mg kg(-1), i.p.) also blocked the impairment of passive avoidance performance caused by the nicotinic receptor antagonist mecamylamine (15 mg kg(-1), i.p.) and the N-methyl-D-aspartate (NMDA) receptor antagonist (+/-)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP; 7.5 mg kg(-1), i.p.), but it failed to affect the deficit caused by the benzodiazepine receptor agonist diazepam (2 mg kg(-1), i.p.). Rhynchophylline significantly reduced the mecamylamine-induced deficit in passive avoidance behaviour, but it failed to attenuate the effects of CPP and diazepam. These results suggest that Uncaria tomentosa total alkaloids exert a beneficial effect on memory impairment induced by the dysfunction of cholinergic systems in the brain and that the effect of the total alkaloids is partly attributed to the oxindole alkaloids tested. Moreover, these findings raised the possibility that the glutamatergic systems are implicated in the anti-amnesic effect of uncarine E.
Bioactive indole alkaloids from the bark of Uncaria guianensis.[Pubmed:10630124]
Planta Med. 1999 Dec;65(8):759-60.
Bioassay-guided fractionation of the EtOH extract of the bark of Uncaria guianensis (Aubl.) Gmel (Rubiaceae) using a yeast-based assay for DNA-damaging agents has furnished the two weakly but selectively active indole alkaloids Uncarine C (1) and uncarine E (2) as the major bioactive constituents in this assay.


