WY-14643 (Pirinixic Acid)PPARα agonist,selective and highly potent CAS# 50892-23-4 |
- JW 480
Catalog No.:BCC6142
CAS No.:1354359-53-7
- Ro 61-8048
Catalog No.:BCC7619
CAS No.:199666-03-0
- 17-ODYA
Catalog No.:BCC6717
CAS No.:34450-18-5
- Metyrapone
Catalog No.:BCC7632
CAS No.:54-36-4
- Flurofamide
Catalog No.:BCC5660
CAS No.:70788-28-2
- p-Chlorophenylalanine
Catalog No.:BCC5689
CAS No.:7424-00-2
Quality Control & MSDS
Number of papers citing our products

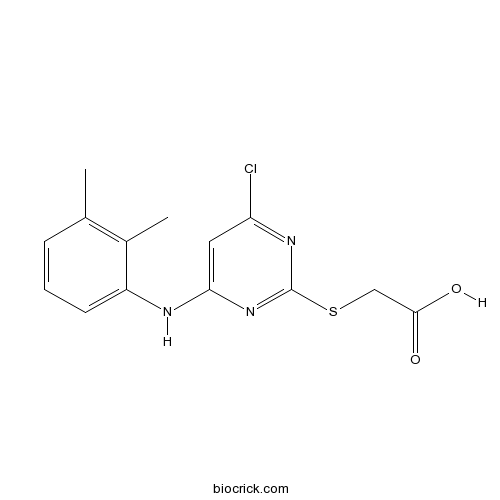
Chemical structure
3D structure
| Cas No. | 50892-23-4 | SDF | Download SDF |
| PubChem ID | 5694 | Appearance | Powder |
| Formula | C14H14ClN3O2S | M.Wt | 323.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (308.83 mM; Need ultrasonic) | ||
| Chemical Name | 2-[4-chloro-6-(2,3-dimethylanilino)pyrimidin-2-yl]sulfanylacetic acid | ||
| SMILES | CC1=C(C(=CC=C1)NC2=CC(=NC(=N2)SCC(=O)O)Cl)C | ||
| Standard InChIKey | SZRPDCCEHVWOJX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective PPARα agonist (EC50 values are 0.63, 32 and > 100 μM at PPARα, PPARγ and PPARδ respectively). Negatively inhibits NF-κB transcriptional activity and decreases the inflammatory response in vitro and in vivo. |

WY-14643 (Pirinixic Acid) Dilution Calculator

WY-14643 (Pirinixic Acid) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0883 mL | 15.4416 mL | 30.8833 mL | 61.7665 mL | 77.2082 mL |
| 5 mM | 0.6177 mL | 3.0883 mL | 6.1767 mL | 12.3533 mL | 15.4416 mL |
| 10 mM | 0.3088 mL | 1.5442 mL | 3.0883 mL | 6.1767 mL | 7.7208 mL |
| 50 mM | 0.0618 mL | 0.3088 mL | 0.6177 mL | 1.2353 mL | 1.5442 mL |
| 100 mM | 0.0309 mL | 0.1544 mL | 0.3088 mL | 0.6177 mL | 0.7721 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
WY-14643, also known as Pirinixic Acid, has an agonistic action as peroxisome proliferator-activated receptor (PPAR). It is shown that aliphatic α-substitution of WY-14643 enhances both PPARα and PPARγ agonism. It has been demonstrated that aliphatic substitution in a-position to the carboxylic acid head group of WY-14643 improves both PPARa and PPARg activity and leads to balanced dual PPARa/g agonists in the lower micromolar range, with a-hexyl pirinixic acid as the most active compound. WY-14,643 can moderately elevate the level of TNFa mRNA in the liver. WY-14,643 stimulates production of low levels of hepatic TNFα by Kupffer cells which acts indirectly as a hepatocyte mitogen.
Reference
Laura Popescu, Oliver Rau, Jark Böttcher, Yvonne Syha, Manfred Schubert-Zsilavecz. Quinoline-Based Derivatives of Pirinixic Acid as Dual PPAR α/γ Agonists. Archiv der Pharmazie. Volume 340, Issue 7, pages 367–371, July 2007
Heiko Zettl, Michaela Dittrich, Ramona Steri, Ewgenij Proschak, Oliver Rau, Dieter Steinhilber, Gisbert Schneider, Michael Lämmerhofer, Manfred Schubert-Zsilavecz. Novel Pirinixic Acids as PPARα Preferential Dual PPARα/γ Agonists. QSAR & Combinatorial Science. Volume 28, Issue 5, pages 576–586, May 2009
Heidi K.Bojes, Dori R.Germolec, Petia Simeonova, Alessandria Bruccoleri, Robert Schoonhoven, Michael I.Luster, Ronald G.Thurman. Antibodies to tumor necrosis factor alpha prevent increases in cell replication in liver due to the potent peroxisome proliferator, WY-14,643. Carcinogenesis (1997) 18 (4): 669-674.
- 1,4-Bis(2-benzoxazolyl)naphthalene
Catalog No.:BCC8423
CAS No.:5089-22-5
- Liensinine diperchlorate
Catalog No.:BCN6336
CAS No.:5088-90-4
- Neoliquiritin
Catalog No.:BCN6663
CAS No.:5088-75-5
- 1,5,6-Trihydroxy-3-methoxyxanthone
Catalog No.:BCN8121
CAS No.:50868-52-5
- Tetramisole HCl
Catalog No.:BCC4735
CAS No.:5086-74-8
- Friedelanol
Catalog No.:BCN5620
CAS No.:5085-72-3
- 1,3,5,6-Tetrahydroxyxanthone
Catalog No.:BCN3453
CAS No.:5084-31-1
- 14,15-Didehydroisoeburnamine
Catalog No.:BCN5619
CAS No.:50838-11-4
- Vortioxetine
Catalog No.:BCC2046
CAS No.:508233-74-7
- Ganoderic acid LM2
Catalog No.:BCN2442
CAS No.:508182-41-0
- Suchilactone
Catalog No.:BCN6752
CAS No.:50816-74-5
- Hastatoside
Catalog No.:BCN6898
CAS No.:50816-24-5
- 1-Acetyl-beta-carboline
Catalog No.:BCN3101
CAS No.:50892-83-6
- Gelsemine
Catalog No.:BCN5804
CAS No.:509-15-9
- Delsoline
Catalog No.:BCN5405
CAS No.:509-18-2
- Aconine
Catalog No.:BCN2394
CAS No.:509-20-6
- Napellonine
Catalog No.:BCN2536
CAS No.:509-24-0
- Strychnine phosphate
Catalog No.:BCC8257
CAS No.:509-42-2
- Mitraphylline
Catalog No.:BCC8213
CAS No.:509-80-8
- Arteannuin B
Catalog No.:BCN5623
CAS No.:50906-56-4
- Toxyloxanthone D
Catalog No.:BCN3070
CAS No.:50906-62-2
- Nemorensine
Catalog No.:BCN2099
CAS No.:50906-96-2
- Taiwanhomoflavone B
Catalog No.:BCN5624
CAS No.:509077-91-2
- 1beta-Hydroxytorilin
Catalog No.:BCN7095
CAS No.:509078-16-4
Anti-obesogenic effects of WY14643 (PPAR-alpha agonist): Hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice.[Pubmed:28711683]
Biochimie. 2017 Sep;140:106-116.
Non-alcoholic fatty liver disease (NAFLD) presents with growing prevalence worldwide, though its pharmacological treatment remains to be established. This study aimed to evaluate the effects of a PPAR-alpha agonist on liver tissue structure, ultrastructure, and metabolism, focusing on gene and protein expression of de novo lipogenesis and gluconeogenesis pathways, in diet-induced obese mice. Male C57BL/6 mice (three months old) received a control diet (C, 10% of lipids, n = 10) or a high-fat diet (HFD, 50% of lipids, n = 10) for ten weeks. These groups were subdivided to receive the treatment (n = 5 per group): C, C-alpha (PPAR-alpha agonist, 2.5 mg/kg/day mixed in the control diet), HFD and HFD-alpha group (PPAR-alpha agonist, 2.5 mg/kg/day mixed in the HFD). The effects were compared with biometrical, biochemical, molecular biology and transmission electron microscopy (TEM) analyses. HFD showed greater body mass (BM) and insulinemia than C, both of which were tackled by the treatment in the HFD-alpha group. Increased hepatic protein expression of glucose-6-phosphatase, CHREBP and gene expression of PEPCK in HFD points to increased gluconeogenesis. Treatment rescued these parameters in the HFD-alpha group, eliciting a reduced hepatic glucose output, confirmed by the smaller GLUT2 expression in HFD-alpha than in HFD. Conversely, favored de novo lipogenesis was found in the HFD group by the increased expression of PPAR-gamma, and its target gene SREBP-1, FAS and GK when compared to C. The treatment yielded a marked reduction in the expression of all lipogenic factors. TEM analyses showed a greater numerical density of mitochondria per area of tissue in treated than in untreated groups, suggesting an increase in beta-oxidation and the consequent NAFLD control. PPAR-alpha activation reduced BM and treated insulin resistance (IR) and NAFLD by increasing the number of mitochondria and reducing hepatic gluconeogenesis and de novo lipogenesis protein and gene expressions in a murine obesity model.
Multifaceted Mechanisms of WY-14643 to Stabilize the Blood-Brain Barrier in a Model of Traumatic Brain Injury.[Pubmed:28603485]
Front Mol Neurosci. 2017 May 26;10:149.
The blood-brain barrier (BBB) is damaged during ischemic insults such as traumatic brain injury or stroke. This contributes to vasogenic edema formation and deteriorate disease outcomes. Enormous efforts are pursued to understand underlying mechanisms of ischemic insults and develop novel therapeutic strategies. In the present study the effects of PPARalpha agonist WY-14643 were investigated to prevent BBB breakdown and reduce edema formation. WY-14643 inhibited barrier damage in a mouse BBB in vitro model of traumatic brain injury based on oxygen/glucose deprivation in a concentration dependent manner. This was linked to changes of the localization of tight junction proteins. Furthermore, WY-14643 altered phosphorylation of kinases ERK1/2, p38, and SAPK/JNK and was able to inhibit proteosomal activity. Moreover, addition of WY-14643 upregulated PAI-1 leading to decreased t-PA activity. Mouse in vivo experiments showed significantly decreased edema formation in a controlled cortical impact model of traumatic brain injury after WY-14643 application, which was not found in PAI-1 knockout mice. Generally, data suggested that WY-14643 induced cellular responses which were dependent as well as independent from PPARalpha mediated transcription. In conclusion, novel mechanisms of a PPARalpha agonist were elucidated to attenuate BBB breakdown during traumatic brain injury in vitro.
PPARalpha-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling.[Pubmed:28558994]
Toxicology. 2017 Jul 15;387:95-107.
Perfluoroalkyl acids (PFAAs) are ubiquitous and persistent environmental contaminants. Compounds such as perfluoroocanoic acid (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonate (PFHxS) are readily found in the tissues of humans and wildlife. While PFOA and PFOS have been the subject of numerous studies since they were first described over a decade ago, less is known about the biological activity of PFHxS and PFNA. Most PFAAs are activators of peroxisome proliferator-activated receptor alpha (PPARalpha), although the biological effects of these compounds are likely mediated by other factors in addition to PPARalpha. To evaluate the effects of PFHxS and PFNA, male wild-type and Pparalpha-null mice were dosed by oral gavage with PFHxS (3 or 10mg/kg/day), PFNA (1 or 3mg/kg/day), or vehicle for 7days, and liver gene expression was evaluated by full-genome microarrays. Gene expression patterns were then compared to historical in-house data for PFOA and PFOS in addition to the experimental hypolipidemic agent, WY-14,643. While WY-14,643 altered most genes in a PPARalpha-dependent manner, approximately 11-24% of regulated genes in PFAA-treated mice were independent of PPARalpha. The possibility that PFAAs regulate gene expression through other molecular pathways was evaluated. Using data available through a microarray database, PFAA gene expression profiles were found to exhibit significant similarity to profiles from mouse tissues exposed to agonists of the constitutive activated receptor (CAR), estrogen receptor alpha (ERalpha), and PPARgamma. Human PPARgamma and ERalpha were activated by all four PFAAs in trans-activation assays from the ToxCast screening program. Predictive gene expression biomarkers showed that PFAAs activate CAR in both genotypes and cause feminization of the liver transcriptome through suppression of signal transducer and activator of transcription 5B (STAT5B). These results indicate that, in addition to activating PPARalpha as a primary target, PFAAs also have the potential to activate CAR, PPARgamma, and ERalpha as well as suppress STAT5B.
Acyl-CoA Thioesterase 1 (ACOT1) Regulates PPARalpha to Couple Fatty Acid Flux With Oxidative Capacity During Fasting.[Pubmed:28607105]
Diabetes. 2017 Aug;66(8):2112-2123.
Hepatic acyl-CoA thioesterase 1 (ACOT1) catalyzes the conversion of acyl-CoAs to fatty acids (FAs) and CoA. We sought to determine the role of ACOT1 in hepatic lipid metabolism in C57Bl/6J male mice 1 week after adenovirus-mediated Acot1 knockdown. Acot1 knockdown reduced liver triglyceride (TG) as a result of enhanced TG hydrolysis and subsequent FA oxidation. In vitro experiments demonstrated that Acot1 knockdown led to greater TG turnover and FA oxidation, suggesting that ACOT1 is important for controlling the rate of FA oxidation. Despite increased FA oxidation, Acot1 knockdown reduced the expression of peroxisome proliferator-activated receptor alpha (PPARalpha) target genes, whereas overexpression increased PPARalpha reporter activity, suggesting ACOT1 regulates PPARalpha by producing FA ligands. Moreover, ACOT1 exhibited partial nuclear localization during fasting and cAMP/cAMP-dependent protein kinase signaling, suggesting local regulation of PPARalpha. As a consequence of increased FA oxidation and reduced PPARalpha activity, Acot1 knockdown enhanced hepatic oxidative stress and inflammation. The effects of Acot1 knockdown on PPARalpha activity, oxidative stress, and inflammation were rescued by supplementation with Wy-14643, a synthetic PPARalpha ligand. We demonstrate through these results that ACOT1 regulates fasting hepatic FA metabolism by balancing oxidative flux and capacity.
Effects of PPARalpha inhibition in head and neck paraganglioma cells.[Pubmed:28594934]
PLoS One. 2017 Jun 8;12(6):e0178995.
Head and neck paragangliomas (HNPGLs) are rare tumors that may cause important morbidity, because of their tendency to infiltrate the skull base. At present, surgery is the only therapeutic option, but radical removal may be difficult or impossible. Thus, effective targets and molecules for HNPGL treatment need to be identified. However, the lack of cellular models for this rare tumor hampers this task. PPARalpha receptor activation was reported in several tumors and this receptor appears to be a promising therapeutic target in different malignancies. Considering that the role of PPARalpha in HNPGLs was never studied before, we analyzed the potential of modulating PPARalpha in a unique model of HNPGL cells. We observed an intense immunoreactivity for PPARalpha in HNPGL tumors, suggesting that this receptor has an important role in HNPGL. A pronounced nuclear expression of PPARalpha was also confirmed in HNPGL-derived cells. The specific PPARalpha agonist WY14643 had no effect on HNPGL cell viability, whereas the specific PPARalpha antagonist GW6471 reduced HNPGL cell viability and growth by inducing cell cycle arrest and caspase-dependent apoptosis. GW6471 treatment was associated with a marked decrease of CDK4, cyclin D3 and cyclin B1 protein expression, along with an increased expression of p21 in HNPGL cells. Moreover, GW6471 drastically impaired clonogenic activity of HNPGL cells, with a less marked effect on cell migration. Notably, the effects of GW6471 on HNPGL cells were associated with the inhibition of the PI3K/GSK3beta/beta-catenin signaling pathway. In conclusion, the PPARalpha antagonist GW6471 reduces HNPGL cell viability, interfering with cell cycle and inducing apoptosis. The mechanisms affecting HNPGL cell viability involve repression of the PI3K/GSK3beta/beta-catenin pathway. Therefore, PPARalpha could represent a novel therapeutic target for HNPGL.
Peroxisome proliferator-activated receptors in the cardiovascular system.[Pubmed:10696077]
Br J Pharmacol. 2000 Mar;129(5):823-34.
Peroxisome proliferator-activated receptor (PPAR)s are a family of three nuclear hormone receptors, PPARalpha, -delta, and -gamma, which are members of the steriod receptor superfamily. The first member of the family (PPARalpha) was originally discovered as the mediator by which a number of xenobiotic drugs cause peroxisome proliferation in the liver. Defined functions for all these receptors, until recently, mainly concerned their ability to regulate energy balance, with PPARalpha being involved in beta-oxidation pathways, and PPARgamma in the differentiation of adipocytes. Little is known about the functions of PPARdelta, though it is the most ubiquitously expressed. Since their discovery, PPARs have been shown to be expressed in monocytes/macrophages, the heart, vascular smooth muscle cells, endothelial cells, and in atherosclerotic lesions. Furthermore, PPARs can be activated by a vast number of compounds including synthetic drugs, of the clofibrate, and anti-diabetic thiazoldinedione classes, polyunsaturated fatty acids, and a number of eicosanoids, including prostaglandins, lipoxygenase products, and oxidized low density lipoprotein. This review will aim to introduce the field of PPAR nuclear hormone receptors, and discuss the discovery and actions of PPARs in the cardiovascular system, as well as the source of potential ligands.
Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta.[Pubmed:9113986]
Proc Natl Acad Sci U S A. 1997 Apr 29;94(9):4312-7.
Fatty acids (FAs) and their derivatives are essential cellular metabolites whose concentrations must be closely regulated. This implies that regulatory circuits exist which can sense changes in FA levels. Indeed, the peroxisome proliferator-activated receptor alpha (PPARalpha) regulates lipid homeostasis and is transcriptionally activated by a variety of lipid-like compounds. It remains unclear as to how these structurally diverse compounds can activate a single receptor. We have developed a novel conformation-based assay that screens activators for their ability to bind to PPARalpha/delta and induce DNA binding. We show here that specific FAs, eicosanoids, and hypolipidemic drugs are ligands for PPARalpha or PPARdelta. Because altered FA levels are associated with obesity, atherosclerosis, hypertension, and diabetes, PPARs may serve as molecular sensors that are central to the development and treatment of these metabolic disorders.


