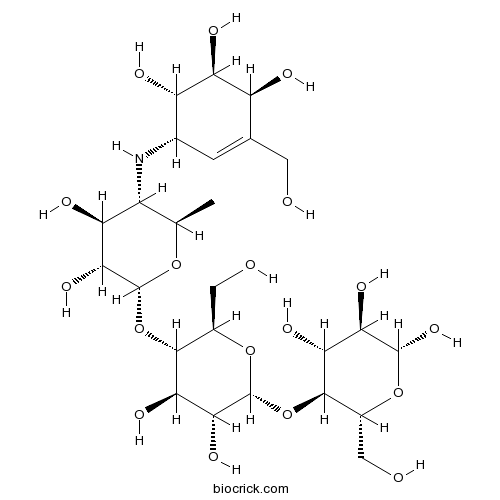
AcarboseAlpha-glucosidase inhibitor CAS# 56180-94-0 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 56180-94-0 | SDF | Download SDF |
| PubChem ID | 441184 | Appearance | Powder |
| Formula | C25H43NO18 | M.Wt | 645.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Bay-g 5421 | ||
| Solubility | H2O : 125 mg/mL (193.62 mM; Need ultrasonic) | ||
| Chemical Name | (2R,3R,4R,5S,6R)-5-[(2R,3R,4R,5S,6R)-5-[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-[[(1S,4S,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino]oxan-2-yl]oxy-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-(hydroxymethyl)oxane-2,3,4-triol | ||
| SMILES | CC1C(C(C(C(O1)OC2C(OC(C(C2O)O)OC3C(OC(C(C3O)O)O)CO)CO)O)O)NC4C=C(C(C(C4O)O)O)CO | ||
| Standard InChIKey | XUFXOAAUWZOOIT-JMPDRRIHSA-N | ||
| Standard InChI | InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(30)15(33)13(8)31)14(32)19(37)24(40-6)43-22-10(5-29)42-25(20(38)17(22)35)44-21-9(4-28)41-23(39)18(36)16(21)34/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12+,13+,14+,15+,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of intestinal α-glucosidase (IC50 = 11 nM). Antidiabetic; inhibits the hydrolysis of complex carbohydrates. |

Acarbose Dilution Calculator

Acarbose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5489 mL | 7.7447 mL | 15.4895 mL | 30.9789 mL | 38.7237 mL |
| 5 mM | 0.3098 mL | 1.5489 mL | 3.0979 mL | 6.1958 mL | 7.7447 mL |
| 10 mM | 0.1549 mL | 0.7745 mL | 1.5489 mL | 3.0979 mL | 3.8724 mL |
| 50 mM | 0.031 mL | 0.1549 mL | 0.3098 mL | 0.6196 mL | 0.7745 mL |
| 100 mM | 0.0155 mL | 0.0774 mL | 0.1549 mL | 0.3098 mL | 0.3872 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Inhibitor of intestinal α-glucosidase (IC50 = 11 nM). Antidiabetic; inhibits the hydrolysis of complex carbohydrates.
- H-Sar-OtBu.HCl
Catalog No.:BCC3336
CAS No.:5616-81-9
- Valrubicin
Catalog No.:BCC5219
CAS No.:56124-62-0
- 4-O-Methylhelichrysetin
Catalog No.:BCN3986
CAS No.:56121-44-9
- Asperglaucide
Catalog No.:BCN5748
CAS No.:56121-42-7
- Parisaponin I
Catalog No.:BCN2835
CAS No.:561007-63-4
- Securinine
Catalog No.:BCN6988
CAS No.:5610-40-2
- Ionomycin calcium salt
Catalog No.:BCC5805
CAS No.:56092-82-1
- Ionomycin free acid
Catalog No.:BCC7261
CAS No.:56092-81-0
- 3-Methoxyshancigusin I
Catalog No.:BCC9002
CAS No.:
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- Hispidin
Catalog No.:BCN3567
CAS No.:56070-89-4
- Sucralose
Catalog No.:BCC4725
CAS No.:56038-13-2
- CGP 13501
Catalog No.:BCC7097
CAS No.:56189-68-5
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
- Torsemide
Catalog No.:BCC4871
CAS No.:56211-40-6
- Methyl lycernuate A
Catalog No.:BCN5749
CAS No.:56218-46-3
- Porson
Catalog No.:BCN5750
CAS No.:56222-03-8
- Cyclo(Tyr-Gly)
Catalog No.:BCN2414
CAS No.:5625-49-0
- Cyclo(Leu-Val)
Catalog No.:BCN2436
CAS No.:5625-50-3
- Hydroxyanigorufone
Catalog No.:BCN7184
CAS No.:56252-02-9
- 2-O-Methylanigorufone
Catalog No.:BCN7180
CAS No.:56252-05-2
- Anigorufone
Catalog No.:BCN7171
CAS No.:56252-32-5
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Aloperine
Catalog No.:BCN8466
CAS No.:56293-29-9
Efficacy and safety of saxagliptin compared with acarbose in Chinese patients with type 2 diabetes mellitus uncontrolled on metformin monotherapy: Results of a Phase IV open-label randomized controlled study (the SMART study).[Pubmed:28296055]
Diabetes Obes Metab. 2017 Nov;19(11):1513-1520.
AIM: To investigate the efficacy, safety and tolerability of saxagliptin compared with Acarbose in Chinese patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy. METHODS: SMART was a 24-week, multicentre, randomized, parallel-group, open-label Phase IV study conducted at 35 sites in China (September 24, 2014 to September 29, 2015). The primary outcome was absolute change from baseline in HbA1c at Week 24. Secondary outcomes assessed at Week 24 included the proportion of patients achieving HbA1c < 7.0%, the proportion of patients with gastrointestinal adverse events (GI AEs), and the proportion of patients achieving HbA1c < 7.0% without GI AEs. Safety and tolerability were also assessed in all patients who received >/=1 dose of study medication. RESULTS: Four-hundred and eighty-eight patients were randomized (1:1) to saxagliptin or Acarbose via a central randomization system (interactive voice/web response system); 241 and 244 patients received saxagliptin and Acarbose, respectively, and 238 and 243 of these had >/=1 pre- and >/=1 post-baseline efficacy values recorded. Saxagliptin was non-inferior to Acarbose for glycaemic control [Week 24 HbA1c change: -0.82% and -0.78%, respectively; difference (95% confidence interval): -0.04 (-0.22, 0.13)%], with similar proportions of patients in both treatment groups achieving HbA1c < 7.0%. However, fewer GI AEs were reported with saxagliptin compared with Acarbose, and a greater number of patients who received saxagliptin achieved HbA1c < 7.0% without GI AEs compared with those receiving Acarbose. CONCLUSION: Both therapies had similar efficacy profiles. However, saxagliptin was associated with fewer GI AEs, suggesting it might be preferential for clinical practice. CLINICAL TRIAL REGISTRATION NUMBER: NCT02243176, clinicaltrials.gov.
Enhanced acarbose production by Streptomyces M37 using a two-stage fermentation strategy.[Pubmed:28234967]
PLoS One. 2017 Feb 24;12(2):e0166985.
In this work, we investigated the effect of pH on Streptomyces M37 growth and its Acarbose biosynthesis ability. We observed that low pH was beneficial for cell growth, whereas high pH favored Acarbose synthesis. Moreover, addition of glucose and maltose to the fermentation medium after 72 h of cultivation promoted Acarbose production. Based on these results, a two-stage fermentation strategy was developed to improve Acarbose production. Accordingly, pH was kept at 7.0 during the first 72 h and switched to 8.0 after that. At the same time, glucose and maltose were fed to increase Acarbose accumulation. With this strategy, we achieved an Acarbose titer of 6210 mg/L, representing an 85.7% increase over traditional batch fermentation without pH control. Finally, we determined that the increased Acarbose production was related to the high activity of glutamate dehydrogenase and glucose 6-phosphate dehydrogenase.
Effectiveness of acarbose in treating elderly patients with diabetes with postprandial hypotension.[Pubmed:28213385]
J Investig Med. 2017 Apr;65(4):772-783.
: Postprandial hypotension (PPH) is a common condition that occurs primarily in elderly patients with type 2 diabetes mellitus (T2DM). This study aimed to assess the effectiveness of Acarbose for PPH; it also investigated possible mechanisms behind PPH development. This single-blind, randomized controlled trial included 91 elderly patients with T2DM, aged between 60 and 80 years, who were inpatients at Beijing Hospital between March 2012 and November 2014. The patients were included into one of three groups: Group A, patients with T2DM without PPH; Group B, patients with T2DM with PPH receiving placebo; and Group C, patients with T2DM with PPH receiving Acarbose. After an overnight fast, patients received a single dose of Acarbose (100 mg) or placebo and then consumed a standardized 450 kcal meal. Blood pressure, glucose levels, heart rate (HR), and catecholamine levels were evaluated. Acarbose ameliorated PPH as determined by significant improvements in the duration and maximal fall in blood pressure (both p<0.001); however, no differences in HR and blood glucose levels were observed. In patients with PPH, blood pressure was correlated with blood glucose and HR variability values (p<0.05). Correlations between epinephrine and glucagon-like peptide-1 with blood pressure in groups A and C were largely lost in group B. Acarbose reduced postprandial blood pressure fluctuations in elderly patients with diabetes. PPH may be related to impaired autonomic nervous system function, reduced catecholamine secretion, and postprandial fluctuations in blood glucose levels. TRIAL REGISTRATION NUMBER: Chinese Clinical Trial Registry ChiCTR-IPR-15006177.
The synergistic effect of maltose enhances the anti-melanogenic activity of acarbose.[Pubmed:28185012]
Arch Dermatol Res. 2017 Apr;309(3):217-223.
Melanocytes play an important role in maintaining epidermal homeostasis by producing melanin and protecting the skin from harmful environmental factors. However, excessive up- or down-regulation of melanin production often causes hyper- or hypo-pigmented disorders, respectively, which affect the patient's quality of life. Therefore, various strategies for modulating melanin levels have been developed by the pharmaceutical and cosmetic industries. We reported previously that voglibose, which is a well-known anti-hyperglycemic agent, could be used as an anti-melanogenic agent by inhibiting alpha-glucosidase activity and reducing tyrosinase protein levels. Of the other representative anti-hyperglycemic agents, Acarbose showed less anti-melanogenic activity despite its potent anti-hyperglycemic efficacy. In this study, we report that Acarbose exhibited considerable anti-melanogenic activity when melanocytes were co-treated with Acarbose and a digestible sugar, such as maltose. Simultaneous treatment with maltose augmented the inhibitory effect of Acarbose on alpha-glucosidase activity by enhancing its stability under physiological conditions, leading to the down-regulation of tyrosinase. These results suggest that the co-treatment of anti-hyperglycemic agents with hydrolysable sugars may be a useful tool for reducing glucosidase-associated melanogenesis as a potent sugar-based anti-melanogenic regimen.
Evaluation of alpha-glucosidase inhibition by using an immobilized assay system.[Pubmed:10993209]
Biol Pharm Bull. 2000 Sep;23(9):1084-7.
The inhibitory effects of natural and synthetic inhibitors on the intestinal membrane-bound hydrolase, alpha-glucosidase (AGH), were evaluated by using an immobilized cyanogen bromide-activated Sepharose 4B support. Immobilized AGH (iAGH) inhibition study by synthetic inhibitors (Acarbose and voglibose) revealed that the magnitude of inhibition differed from that in the free AGH (fAGH) study: IC50 value of Acarbose in iAGH-maltase assay system, 340-430 nM; fAGH, 11 nM. iAGH-maltase inhibition by both inhibitors was influenced by blocking reagents with different functional groups (COOH, OH, CH3, and NH2 groups). On the other hand, significant iAGH-sucrase inhibitory activity was observed only when using the negatively charged support induced by 0.1 M beta-alanine. The Km values obtained in the iAGH assay system were similar to those from the fAGH method. With natural inhibitors, the iAGH-sucrase inhibitory activity of D-Xylose, with in vivo glucose suppression, increased twice compared to that in fAGH. Green tea extract gave almost the same inhibition for both AGH assay systems.
Acarbose. An update of its pharmacology and therapeutic use in diabetes mellitus.[Pubmed:7510610]
Drugs. 1993 Dec;46(6):1025-54.
Acarbose delays digestion of complex carbohydrates and disaccharides to absorbable monosaccharides, by reversibly inhibiting alpha-glucosidases within the intestinal brush border, thereby attenuating postprandial blood glucose peaks. Clinical trials have demonstrated that Acarbose generally improves glycaemic control in patients with non-insulin-dependent diabetes mellitus (NIDDM) managed with diet alone, or with other antidiabetic therapy, as evidenced by decreased postprandial plasma glucose and glycosylated haemoglobin levels. It does not appear to directly alter insulin resistance, but may lower postprandial plasma insulin levels. Fasting plasma glucose, triglyceride and/or cholesterol levels may also be decreased. Acarbose also improved metabolic control in patients with insulin-dependent diabetes mellitus (IDDM), frequently decreasing insulin requirements, although further studies are required in this indication. Improved metabolic control appears to delay or prevent long term vascular complications of diabetes, and indeed, Acarbose appeared to inhibit development of such complications in preliminary animal studies, but this finding requires confirmation in clinical studies. While Acarbose seldom causes systemic adverse effects, it is associated with a high incidence of gastrointestinal disturbances such as flatulence, abdominal distension, borborygmus and diarrhoea, caused by fermentation of unabsorbed carbohydrates. However, these symptoms tend to subside with continued treatment and adherence to an appropriate diet. Thus, Acarbose appears to be a worthwhile adjunctive therapeutic option for patients with NIDDM inadequately managed by diet alone, or with pharmacological therapy, and possibly also for patients with IDDM. However, further long term efficacy and tolerability data are required, particularly in the latter indication.
Inhibition of disaccharide digestion in rat intestine by the alpha-glucosidase inhibitor acarbose (BAY g 5421).[Pubmed:6754513]
Digestion. 1982;23(4):232-8.
Administration of the alpha-glucosidase inhibitor, Acarbose (BAY g 5421), to rats together with a sucrose load results in a marked retardation of sucrose digestion. The carbohydrate content of the small intestine is dose dependently increased; the time needed for the absorption is doubled. In the large intestine significant amounts of carbohydrate can be found only after administration of high doses of Acarbose (2-4 mg/kg p.o.). In oral sucrose and maltose loading tests the blood glucose increase is dose dependently reduced by Acarbose (ED50, 1 or 12 mg/kg, respectively). In perfused jejunal loops of rats, Acarbose inhibits the absorption of sucrose (4 g/l) and maltose (1 and 2 g/l), the IC50 values being 3.2, 36, and 57 micrograms/ml, respectively. The data indicate that Acarbose effectively inhibits sucrose digestion. It is 10-20 times less effective with maltose as a substrate. Slight malabsorption is induced by Acarbose only in doses higher than the ED50.


