HedychenoneCAS# 56324-54-0 |
Quality Control & MSDS
Number of papers citing our products

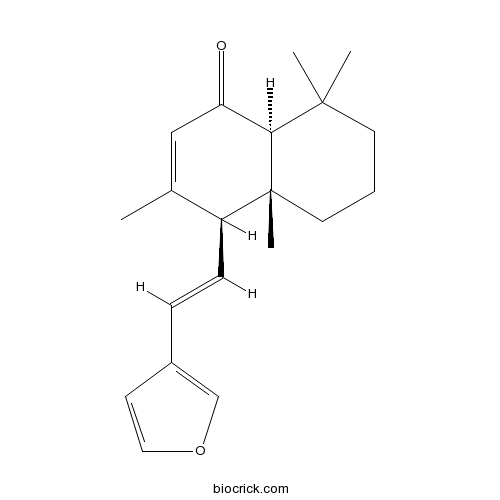
Chemical structure

3D structure
| Cas No. | 56324-54-0 | SDF | Download SDF |
| PubChem ID | 12067184 | Appearance | Powder |
| Formula | C20H26O2 | M.Wt | 298.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4S,4aR,8aS)-4-[(E)-2-(furan-3-yl)ethenyl]-3,4a,8,8-tetramethyl-5,6,7,8a-tetrahydro-4H-naphthalen-1-one | ||
| SMILES | CC1=CC(=O)C2C(CCCC2(C1C=CC3=COC=C3)C)(C)C | ||
| Standard InChIKey | MXTCKNHXBBXULO-ZJDHVTHPSA-N | ||
| Standard InChI | InChI=1S/C20H26O2/c1-14-12-17(21)18-19(2,3)9-5-10-20(18,4)16(14)7-6-15-8-11-22-13-15/h6-8,11-13,16,18H,5,9-10H2,1-4H3/b7-6+/t16-,18-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hedychenone has anti-inflammatory activity, it also shows potent in vitro cytotoxic activity against cancerous cells. |
| Targets | Immunology & Inflammation related |
| In vitro | Synthesis, cytotoxic activity and structure-activity relationships of hedychenone analogues.[Pubmed: 20303755]Bioorg Med Chem Lett. 2010 Apr 15;20(8):2525-8.Hedychenone, a plant-derived labdane diterpenoid, showed potent in vitro cytotoxic activity against cancerous cells. |
| Structure Identification | Journal of Planar Chromatography - Modern TLC, 2007 , 20 (1) :73-74.A new, convenient method for quantitative analysis of hedychenone, an anti-inflammatory compound in the rhizomes of Hedychium spicatum (Buch-Hem)[Reference: WebLink]Plants of the Hedychium genus are perennial rhizomatus plants belonging to the Zingiberaceae family. Extracts of Zingiberacaea have long been used in traditional medicine.
|

Hedychenone Dilution Calculator

Hedychenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3512 mL | 16.756 mL | 33.5121 mL | 67.0241 mL | 83.7802 mL |
| 5 mM | 0.6702 mL | 3.3512 mL | 6.7024 mL | 13.4048 mL | 16.756 mL |
| 10 mM | 0.3351 mL | 1.6756 mL | 3.3512 mL | 6.7024 mL | 8.378 mL |
| 50 mM | 0.067 mL | 0.3351 mL | 0.6702 mL | 1.3405 mL | 1.6756 mL |
| 100 mM | 0.0335 mL | 0.1676 mL | 0.3351 mL | 0.6702 mL | 0.8378 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Conocarpan acetate
Catalog No.:BCN7584
CAS No.:56319-04-1
- Moracin M
Catalog No.:BCN3292
CAS No.:56317-21-6
- 5,6,7,8-Tetramethoxycoumarin
Catalog No.:BCN5752
CAS No.:56317-15-8
- Kaempferol 3-O-(6''-galloyl)-beta-D-glucopyranoside
Catalog No.:BCN1416
CAS No.:56317-05-6
- Trimethylapigenin
Catalog No.:BCN8081
CAS No.:5631-70-9
- 3-(2,4-Dihydroxyphenyl)propionic acid
Catalog No.:BCN5751
CAS No.:5631-68-5
- Fluoxetine HCl
Catalog No.:BCC1191
CAS No.:56296-78-7
- Aloperine
Catalog No.:BCN8466
CAS No.:56293-29-9
- Uncarine C
Catalog No.:BCC8261
CAS No.:5629-60-7
- Anigorufone
Catalog No.:BCN7171
CAS No.:56252-32-5
- 2-O-Methylanigorufone
Catalog No.:BCN7180
CAS No.:56252-05-2
- Hydroxyanigorufone
Catalog No.:BCN7184
CAS No.:56252-02-9
- Oxybutynin
Catalog No.:BCC3833
CAS No.:5633-20-5
- Capillarisin
Catalog No.:BCN2461
CAS No.:56365-38-9
- Tirotundin
Catalog No.:BCN5754
CAS No.:56377-67-4
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Netilmicin Sulfate
Catalog No.:BCC4683
CAS No.:56391-57-2
- Metoprolol Tartrate
Catalog No.:BCC4330
CAS No.:56392-17-7
- UK-5099
Catalog No.:BCC2021
CAS No.:56396-35-1
- Hop-17(21)-en-3-ol
Catalog No.:BCN5755
CAS No.:564-14-7
- Sclareolide
Catalog No.:BCC6492
CAS No.:564-20-5
- Doxycycline
Catalog No.:BCN2397
CAS No.:564-25-0
- 11α-Hydroxyandrost-4-ene-3,17-dione
Catalog No.:BCC8432
CAS No.:564-33-0
- Hinokiol
Catalog No.:BCN5759
CAS No.:564-73-8
Synthesis, cytotoxic activity and structure-activity relationships of hedychenone analogues.[Pubmed:20303755]
Bioorg Med Chem Lett. 2010 Apr 15;20(8):2525-8.
Hedychenone, a plant-derived labdane diterpenoid, showed potent in vitro cytotoxic activity against cancerous cells. In the present study, a series of analogues have been synthesized by modification of the furanoid ring, double bond and the vinylic methyl functionality of this natural product lead and evaluated for their cytotoxic activities against human cancer cell lines. The structures of the target compounds were established by IR, (1)H NMR and mass spectral analysis. Majority of the analogues displayed potent activity than the parent compound, Hedychenone. Preliminary structure-activity relationship studies indicated that furanoid ring has a greater impact on cytotoxicity than that of the decalone nucleus. However, dimerization through C-8 significantly enhanced the cytotoxic activity of the Hedychenone.


