TrilobatinCAS# 4192-90-9 |
Quality Control & MSDS
Number of papers citing our products

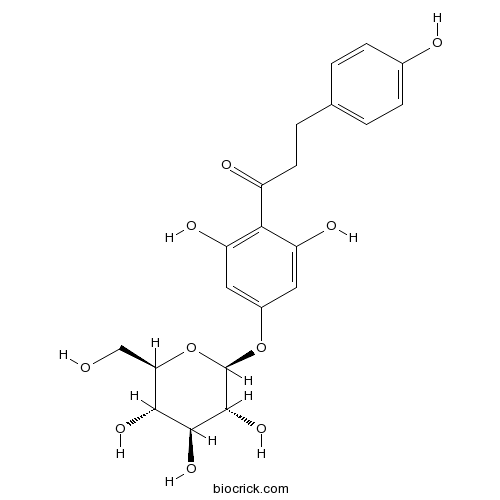
Chemical structure

3D structure
| Cas No. | 4192-90-9 | SDF | Download SDF |
| PubChem ID | 6451798 | Appearance | White powder |
| Formula | C21H24O10 | M.Wt | 436.4 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Synonyms | Phloretin 4'-glucoside; p-Phloridzin; p-Phlorizin; Prunin dihydrochalcone | ||
| Solubility | Soluble in ethan | ||
| Chemical Name | 1-[2,6-dihydroxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-3-(4-hydroxyphenyl)propan-1-one | ||
| SMILES | C1=CC(=CC=C1CCC(=O)C2=C(C=C(C=C2O)OC3C(C(C(C(O3)CO)O)O)O)O)O | ||
| Standard InChIKey | GSTCPEBQYSOEHV-QNDFHXLGSA-N | ||
| Standard InChI | InChI=1S/C21H24O10/c22-9-16-18(27)19(28)20(29)21(31-16)30-12-7-14(25)17(15(26)8-12)13(24)6-3-10-1-4-11(23)5-2-10/h1-2,4-5,7-8,16,18-23,25-29H,3,6,9H2/t16-,18-,19+,20-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Trilobatin has anti-oxidant, and anti-inflammatory effects, it can increase superoxide dismutase (SOD) activity, and it potentially inhibits the lipopolysaccharide (LPS)-induced inflammatory response by suppressing the NF-κB signaling pathway. Trilobatin shows a strong inhibitory activity against α-glucosidase and a moderate inhibitory activity against α-amylase for management of postprandial hyperglycemia with less side effect. |
| Targets | TNF-α | IL Receptor | IkB | NF-kB | p65 | IKK | SOD |
| In vitro | Trilobatin attenuates the LPS-mediated inflammatory response by suppressing the NF-κB signaling pathway.[Pubmed: 25053100 ]Food Chem. 2015 Jan 1;166:609-15.We investigated the anti-inflammatory effect of Trilobatin, the flavonoid isolated from the leaves of Lithocarpus polystachyus Rehd, as well as the underlying molecular mechanisms. Antioxidant activities of three dihydrochalcone glucosides from leaves of Lithocarpus pachyphyllus.[Pubmed: 15813365]Z Naturforsch C. 2004 Jul-Aug;59(7-8):481-4.In vitro antioxidant activities of three sweet dihydrochalcone glucosides from the leaves of Lithocarpus pachyphyllus (Kurz) Rehd. (Fagaceae), Trilobatin 2"-acetate (1), phloridzin (2) and Trilobatin (3), were investigated. |
| Kinase Assay | Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes.[Reference: WebLink]Food Chemistry, 2012, 130(2):261-266.The chemical structure of the sweet compound from Lithocarpus polystachyus Rehd was identified as Trilobatin on the basis of HPLC, EIS-MS and NMR analyses. |

Trilobatin Dilution Calculator

Trilobatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2915 mL | 11.4574 mL | 22.9148 mL | 45.8295 mL | 57.2869 mL |
| 5 mM | 0.4583 mL | 2.2915 mL | 4.583 mL | 9.1659 mL | 11.4574 mL |
| 10 mM | 0.2291 mL | 1.1457 mL | 2.2915 mL | 4.583 mL | 5.7287 mL |
| 50 mM | 0.0458 mL | 0.2291 mL | 0.4583 mL | 0.9166 mL | 1.1457 mL |
| 100 mM | 0.0229 mL | 0.1146 mL | 0.2291 mL | 0.4583 mL | 0.5729 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isopropyl 4-Hydroxybenzoate
Catalog No.:BCN8409
CAS No.:4191-73-5
- Nitrocefin
Catalog No.:BCC6544
CAS No.:41906-86-9
- PYR-41
Catalog No.:BCC4470
CAS No.:418805-02-4
- 2,3-DCPE hydrochloride
Catalog No.:BCC2384
CAS No.:418788-90-6
- Bezafibrate
Catalog No.:BCC4639
CAS No.:41859-67-0
- Alatamine
Catalog No.:BCN3100
CAS No.:41855-33-8
- Mangiferolic acid
Catalog No.:BCN4636
CAS No.:4184-34-3
- Hesperetin-7-methyl ether
Catalog No.:BCN8501
CAS No.:
- Junicedric acid
Catalog No.:BCN7660
CAS No.:41787-69-3
- Hypecorinine
Catalog No.:BCN3298
CAS No.:41787-57-9
- H-Leu-pNA.HCl
Catalog No.:BCC2972
CAS No.:4178-93-2
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- N1-Methyl-4-nitrobenzene-1,2-diamine
Catalog No.:BCC9070
CAS No.:41939-61-1
- MLR 1023
Catalog No.:BCC6232
CAS No.:41964-07-2
- Pinocembrin chalcone
Catalog No.:BCN7223
CAS No.:4197-97-1
- Glabranin
Catalog No.:BCN5480
CAS No.:41983-91-9
- (S)-(-)-Propranolol hydrochloride
Catalog No.:BCC6809
CAS No.:4199-10-4
- 5-Chloro-1,10-phenanthroline
Catalog No.:BCC3713
CAS No.:4199-89-7
- Dihydropashanone
Catalog No.:BCN4635
CAS No.:41997-41-5
- Antitumor Compound 1
Catalog No.:BCC5397
CAS No.:420126-30-3
- Fenofibric acid
Catalog No.:BCC8982
CAS No.:42017-89-0
- Estriol 17-sulfate
Catalog No.:BCN2237
CAS No.:42028-21-7
- Clonidine HCl
Catalog No.:BCC4325
CAS No.:4205-91-8
- Mezlocillin Sodium
Catalog No.:BCC4678
CAS No.:42057-22-7
Trilobatin attenuates the LPS-mediated inflammatory response by suppressing the NF-kappaB signaling pathway.[Pubmed:25053100]
Food Chem. 2015 Jan 1;166:609-15.
We investigated the anti-inflammatory effect of Trilobatin, the flavonoid isolated from the leaves of Lithocarpus polystachyus Rehd, as well as the underlying molecular mechanisms. Treatment with Trilobatin (0.005-5 muM) dose-dependently inhibited the lipopolysaccharide (LPS)-induced mRNA expression and secretion of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNFalpha), interleukin-1beta (IL-1beta) and interleukin-6 (IL-6), in RAW 264.7 macrophages. However, no further inhibition was detected when the concentration of Trilobatin was increased to 50 muM. Western blot analysis confirmed that the mechanism of the anti-inflammatory effect was correlated with the inhibition of LPS-induced inhibitor of nuclear factor-kappa B alpha (IkappaBalpha) degradation and nuclear factor-kappa B (NF-kappaB) p65 phosphorylation. In addition, Trilobatin also showed a significant inhibition of LPS-induced TNFalpha and IL-6 at both the mRNA and protein levels in a mouse model. Our results suggest that Trilobatin potentially inhibits the LPS-induced inflammatory response by suppressing the NF-kappaB signaling pathway.
Antioxidant activities of three dihydrochalcone glucosides from leaves of Lithocarpus pachyphyllus.[Pubmed:15813365]
Z Naturforsch C. 2004 Jul-Aug;59(7-8):481-4.
In vitro antioxidant activities of three sweet dihydrochalcone glucosides from the leaves of Lithocarpus pachyphyllus (Kurz) Rehd. (Fagaceae), Trilobatin 2"-acetate (1), phloridzin (2) and Trilobatin (3), were investigated. The IC50 (50% inhibitory concentration) values for compounds 1-3 of lipid peroxidation in rat liver homogenate were 261, 28, 88 microM, respectively. Compounds 1-3 increased superoxide dismutase (SOD) activity with EC50 (50% effective concentration) values of 575, 167, 128 microM, and glutathione peroxidase (GSH-Px) activity with EC50 values of 717, 347, 129 microM, respectively, and showed only weak DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity.


