Pinocembrin chalconeCAS# 4197-97-1 |
Quality Control & MSDS
Number of papers citing our products

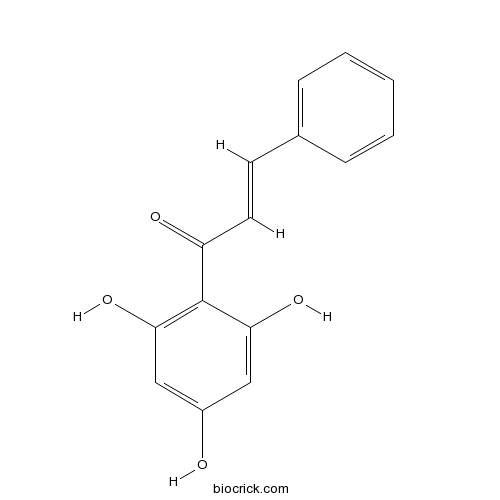
Chemical structure

3D structure
| Cas No. | 4197-97-1 | SDF | Download SDF |
| PubChem ID | 6474295 | Appearance | Powder |
| Formula | C15H12O4 | M.Wt | 256.25 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one | ||
| SMILES | C1=CC=C(C=C1)C=CC(=O)C2=C(C=C(C=C2O)O)O | ||
| Standard InChIKey | LOYXTWZXLWHMBX-VOTSOKGWSA-N | ||
| Standard InChI | InChI=1S/C15H12O4/c16-11-8-13(18)15(14(19)9-11)12(17)7-6-10-4-2-1-3-5-10/h1-9,16,18-19H/b7-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pinocembrin chalcone has tyrosinase inhibitory activity.It also shows antimutagenic effect, which is mainly due to the inhibition of the first step of enzymatic activation of heterocyclic amines. Pinocembrin chalcone shows antimicrobial activity against the antibiotic susceptible NG strain WHO V ; it also displays activity against Candida albicans with a minimal inhibitory concentration value of 100 microg/mL. |
| Targets | Tyrosinase | Calcium Channel | Antifection |
| In vitro | Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae.[Pubmed: 20921932 ]Sex Transm Dis. 2011 Feb;38(2):82-8The successful treatment of Neisseria gonorrhoeae (NG) infections is increasingly problematic because of the resistance of this pathogen to multiple antimicrobial agents. This development underscores the need for new antimicrobial sources. In the current study, 21 crude methanol extracts, from 19 plants used in Colombian traditional medicine for cutaneous infections, were screened for antimicrobial activity against NG.
Synthesis and evaluation of 2',4',6'-trihydroxychalcones as a new class of tyrosinase inhibitors.[Pubmed: 17267225 ]Bioorg Med Chem. 2007 Mar 15;15(6):2396-402.
Structural analysis of a novel antimutagenic compound, 4-Hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines.[Pubmed: 11410007]J Agric Food Chem. 2001 Jun;49(6):3046-50.
|
| Kinase Assay | Antifungal activity of benzoic acid derivatives from Piper lanceaefolium.[Pubmed: 11809068]J Nat Prod. 2002 Jan;65(1):62-4.The aim of this study was to determine the antifungal activity of Drimenol (1) and its synthetic derivatives, nordrimenone (2), drimenyl acetate (3), and drimenyl-epoxy-acetate (4), and to establish a possible mechanism of action for Drimenol. |
| Structure Identification | Biosci Biotechnol Biochem. 2009 Jul;73(7):1679-82.Pinostrobin from Boesenbergia pandurata is an inhibitor of Ca2+-signal-mediated cell-cycle regulation in the yeast Saccharomyces cerevisiae.[Pubmed: 19584530]
|

Pinocembrin chalcone Dilution Calculator

Pinocembrin chalcone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9024 mL | 19.5122 mL | 39.0244 mL | 78.0488 mL | 97.561 mL |
| 5 mM | 0.7805 mL | 3.9024 mL | 7.8049 mL | 15.6098 mL | 19.5122 mL |
| 10 mM | 0.3902 mL | 1.9512 mL | 3.9024 mL | 7.8049 mL | 9.7561 mL |
| 50 mM | 0.078 mL | 0.3902 mL | 0.7805 mL | 1.561 mL | 1.9512 mL |
| 100 mM | 0.039 mL | 0.1951 mL | 0.3902 mL | 0.7805 mL | 0.9756 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MLR 1023
Catalog No.:BCC6232
CAS No.:41964-07-2
- N1-Methyl-4-nitrobenzene-1,2-diamine
Catalog No.:BCC9070
CAS No.:41939-61-1
- Trilobatin
Catalog No.:BCN5479
CAS No.:4192-90-9
- Isopropyl 4-Hydroxybenzoate
Catalog No.:BCN8409
CAS No.:4191-73-5
- Nitrocefin
Catalog No.:BCC6544
CAS No.:41906-86-9
- PYR-41
Catalog No.:BCC4470
CAS No.:418805-02-4
- 2,3-DCPE hydrochloride
Catalog No.:BCC2384
CAS No.:418788-90-6
- Bezafibrate
Catalog No.:BCC4639
CAS No.:41859-67-0
- Alatamine
Catalog No.:BCN3100
CAS No.:41855-33-8
- Mangiferolic acid
Catalog No.:BCN4636
CAS No.:4184-34-3
- Hesperetin-7-methyl ether
Catalog No.:BCN8501
CAS No.:
- Junicedric acid
Catalog No.:BCN7660
CAS No.:41787-69-3
- Glabranin
Catalog No.:BCN5480
CAS No.:41983-91-9
- (S)-(-)-Propranolol hydrochloride
Catalog No.:BCC6809
CAS No.:4199-10-4
- 5-Chloro-1,10-phenanthroline
Catalog No.:BCC3713
CAS No.:4199-89-7
- Dihydropashanone
Catalog No.:BCN4635
CAS No.:41997-41-5
- Antitumor Compound 1
Catalog No.:BCC5397
CAS No.:420126-30-3
- Fenofibric acid
Catalog No.:BCC8982
CAS No.:42017-89-0
- Estriol 17-sulfate
Catalog No.:BCN2237
CAS No.:42028-21-7
- Clonidine HCl
Catalog No.:BCC4325
CAS No.:4205-91-8
- Mezlocillin Sodium
Catalog No.:BCC4678
CAS No.:42057-22-7
- Atractylenolide III acetate
Catalog No.:BCC9147
CAS No.:
- d[Leu4,Lys8]-VP
Catalog No.:BCC5981
CAS No.:42061-33-6
- 2 CTC
Catalog No.:BCC2571
CAS No.:42074-68-0
Antifungal activity of benzoic acid derivatives from Piper lanceaefolium.[Pubmed:11809068]
J Nat Prod. 2002 Jan;65(1):62-4.
Bioactivity-guided fractionation of a methanol extract from the leaves of Piper lanceaefolium resulted in the isolation of four new benzoic acid derivatives (1-4), together with taboganic acid, pinocembrin, and Pinocembrin chalcone. Lanceaefolic acid methyl ester (3) and Pinocembrin chalcone displayed activity against Candida albicans with a minimal inhibitory concentration value of 100 microg/mL in both cases.
Pinostrobin from Boesenbergia pandurata is an inhibitor of Ca2+-signal-mediated cell-cycle regulation in the yeast Saccharomyces cerevisiae.[Pubmed:19584530]
Biosci Biotechnol Biochem. 2009 Jul;73(7):1679-82. Epub 2009 Jul 7.
Upon searching plant extracts for inhibitors of the Ca(2+) signaling pathway using the zds1Delta-yeast proliferation based assay, a crude rhizome extract of Boesenbergia pandurata was found to be strongly positive, and from this extract pinostrobin, alpinetin, and Pinocembrin chalcone were isolated as active components. Further biochemical experiments confirmed that pinostrobin possesses inhibitory activity on the Ca(2+) signals involved in the control of G2/M phase cell cycle progression in Saccharomyces cerevisiae.
Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae.[Pubmed:20921932]
Sex Transm Dis. 2011 Feb;38(2):82-8.
BACKGROUND: The successful treatment of Neisseria gonorrhoeae (NG) infections is increasingly problematic because of the resistance of this pathogen to multiple antimicrobial agents. This development underscores the need for new antimicrobial sources. In the current study, 21 crude methanol extracts, from 19 plants used in Colombian traditional medicine for cutaneous infections, were screened for antimicrobial activity against NG. METHODS: Extracts were screened by disc susceptibility assay. In addition, the minimum inhibitory concentrations of active compounds from P. lanceaefolium were assayed using a panel of 26 NG strains comprising 12 antibiotic-resistant phenotypes. RESULTS: In all, 71% of the crude extracts exhibited antibacterial activity against the antibiotic susceptible NG strain WHO V, whereas 10% of the extracts inhibited penicillinase-producing NG strain GC1-182. The crude extract of Piper lanceaefolium was the only extract to show significant activity without ultraviolet (UV) light activation. Preliminary screening identified 3 compounds in this plant possessing antimicrobial activity: the flavonoids 5,7-dihydroxyflavanone (pinocembrin), 2',4',6'-trihydroxychalcone (Pinocembrin chalcone), and the prenylated benzoic acid derivative cyclolanceaefolic acid methyl ester. Pinocembrin and Pinocembrin chalcone inhibited 100% of the NG panel at 64 mug/mL and 128 mug/mL, respectively, whereas cyclolanceaefolic acid methyl ester inhibited 44% of the strains at 128 mug/mL. CONCLUSIONS: This is the first report of the antibacterial activity of Columbian plants against NG. The activity of the 2 flavonoids, pinocembrin, and Pinocembrin chalcone, toward both susceptible and resistant NG strains makes them promising candidates for further research.
Synthesis and evaluation of 2',4',6'-trihydroxychalcones as a new class of tyrosinase inhibitors.[Pubmed:17267225]
Bioorg Med Chem. 2007 Mar 15;15(6):2396-402.
In this study, we synthesized a series of hydroxychalcones and examined their tyrosinase inhibitory activity. The results showed that 2',4',6'-trihydroxychalcone (1), 2,2',3,4',6'-pentahydroxychalcone (4), 2',3,4,4',5,6'-hexahydroxychalcone (5), 2',4',6'-trihydroxy- 3,4-dimethoxychalcone (9) and 2,2',4,4',6'-pentahydroxychalcone (15) exhibited high inhibitory effects on tyrosinase with respect to l-tyrosine as a substrate. By the structure-activity relationship study, it was suggested that the 2',4',6'-trihydroxyl substructure in the chalcone skeleton were efficacious for the inhibition of tyrosinase activity. And also, the catechol structure on B-ring of chalcones was not advantageous for the inhibitory potency. Furthermore, 15 (IC(50)=1microM) was found to show the highest activity out of a set of 15 hydroxychalcones, even better than both 2,2',4,4'-tetrahydroxychalcone (13, IC(50)=5microM) and kojic acid (16, IC(50)=12microM), which were known as potent tyrosinase inhibitors. Kinetic study revealed that 15 acts as a competitive inhibitor of tyrosinase with K(i) value of 3.1microM.
Structural analysis of a novel antimutagenic compound, 4-Hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines.[Pubmed:11410007]
J Agric Food Chem. 2001 Jun;49(6):3046-50.
Six compounds were isolated from fresh rhizomes of fingerroot (Boesenbergia pandurata Schult.) as strong antimutagens toward 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) in Salmonella typhimurium TA98. These compounds were 2',4',6'-trihydroxychalcone (Pinocembrin chalcone; 1), 2',4'-dihydroxy-6'-methoxychalcone (cardamonin; 2), 5,7-dihydroxyflavanone (pinocembrin; 3), 5-hydroxy-7-methoxyflavanone (pinostrobin; 4), (2,4,6-trihydroxyphenyl)-[3'-methyl-2'-(3' '-methylbut-2' '-enyl)-6'-phenylcyclohex-3'-enyl]methanone (5), and (2,6-dihydroxy-4-methoxyphenyl)-[3'-methyl-2'-(3' '-methylbut-2' '-enyl)-6'-phenylcyclohex-3'-enyl]methanone (panduratin A; 6). Compound 5 was a novel compound (tentatively termed 4-hydroxypanduratin A), and 1 was not previously reported in this plant, whereas 2-4 and 6 were known compounds. The antimutagenic IC(50) values of compounds 1-6 were 5.2 +/- 0.4, 5.9 +/- 0.7, 6.9 +/- 0.8, 5.3 +/- 1.0, 12.7 +/- 0.7, and 12.1 +/- 0.8 microM in the preincubation mixture, respectively. They also similarly inhibited the mutagenicity of 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). All of them strongly inhibited the N-hydroxylation of Trp-P-2. Thus, the antimutagenic effect of compounds 1-6 was mainly due to the inhibition of the first step of enzymatic activation of heterocyclic amines.


