GlabraninCAS# 41983-91-9 |
Quality Control & MSDS
Number of papers citing our products

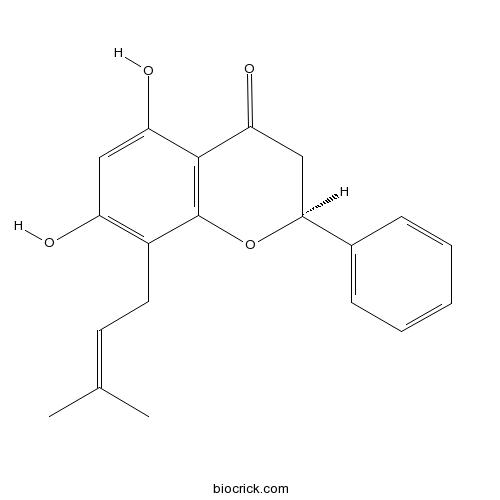
Chemical structure

3D structure
| Cas No. | 41983-91-9 | SDF | Download SDF |
| PubChem ID | 124049 | Appearance | Powder |
| Formula | C20H20O4 | M.Wt | 324.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-5,7-dihydroxy-8-(3-methylbut-2-enyl)-2-phenyl-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=C(C2=C1OC(CC2=O)C3=CC=CC=C3)O)O)C | ||
| Standard InChIKey | DAWSYIQAGQMLFS-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C20H20O4/c1-12(2)8-9-14-15(21)10-16(22)19-17(23)11-18(24-20(14)19)13-6-4-3-5-7-13/h3-8,10,18,21-22H,9,11H2,1-2H3/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Glabranine exerts a dose-dependent inhibitory effect in vitro on the dengue virus, it presents 70% inhibition on the dengue virus at a concentration of 25 microM. 2. Glabranin shows significant activities on DPPH free radical with the IC50 value of 240.20 ug/ml. 3. Glabranin or a derivative thereof could be used to stimulate hair growth. 4. Glabranin has antimicrobial activity. |
| Targets | Antifection |

Glabranin Dilution Calculator

Glabranin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0826 mL | 15.4131 mL | 30.8261 mL | 61.6523 mL | 77.0654 mL |
| 5 mM | 0.6165 mL | 3.0826 mL | 6.1652 mL | 12.3305 mL | 15.4131 mL |
| 10 mM | 0.3083 mL | 1.5413 mL | 3.0826 mL | 6.1652 mL | 7.7065 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6165 mL | 1.233 mL | 1.5413 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6165 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pinocembrin chalcone
Catalog No.:BCN7223
CAS No.:4197-97-1
- MLR 1023
Catalog No.:BCC6232
CAS No.:41964-07-2
- N1-Methyl-4-nitrobenzene-1,2-diamine
Catalog No.:BCC9070
CAS No.:41939-61-1
- Trilobatin
Catalog No.:BCN5479
CAS No.:4192-90-9
- Isopropyl 4-Hydroxybenzoate
Catalog No.:BCN8409
CAS No.:4191-73-5
- Nitrocefin
Catalog No.:BCC6544
CAS No.:41906-86-9
- PYR-41
Catalog No.:BCC4470
CAS No.:418805-02-4
- 2,3-DCPE hydrochloride
Catalog No.:BCC2384
CAS No.:418788-90-6
- Bezafibrate
Catalog No.:BCC4639
CAS No.:41859-67-0
- Alatamine
Catalog No.:BCN3100
CAS No.:41855-33-8
- Mangiferolic acid
Catalog No.:BCN4636
CAS No.:4184-34-3
- Hesperetin-7-methyl ether
Catalog No.:BCN8501
CAS No.:
- (S)-(-)-Propranolol hydrochloride
Catalog No.:BCC6809
CAS No.:4199-10-4
- 5-Chloro-1,10-phenanthroline
Catalog No.:BCC3713
CAS No.:4199-89-7
- Dihydropashanone
Catalog No.:BCN4635
CAS No.:41997-41-5
- Antitumor Compound 1
Catalog No.:BCC5397
CAS No.:420126-30-3
- Fenofibric acid
Catalog No.:BCC8982
CAS No.:42017-89-0
- Estriol 17-sulfate
Catalog No.:BCN2237
CAS No.:42028-21-7
- Clonidine HCl
Catalog No.:BCC4325
CAS No.:4205-91-8
- Mezlocillin Sodium
Catalog No.:BCC4678
CAS No.:42057-22-7
- Atractylenolide III acetate
Catalog No.:BCC9147
CAS No.:
- d[Leu4,Lys8]-VP
Catalog No.:BCC5981
CAS No.:42061-33-6
- 2 CTC
Catalog No.:BCC2571
CAS No.:42074-68-0
- Lucialdehyde A
Catalog No.:BCN2449
CAS No.:420781-84-6
Antiviral effect of flavonoids on the dengue virus.[Pubmed:10685103]
Phytother Res. 2000 Mar;14(2):89-92.
In the present study we analysed the possible antiviral effect on dengue viruses of different flavonoids extracted and identified at the Chemistry Institute, UNAM, from the Mexican plants Tephrosia madrensis, Tephrosia viridiflora and Tephrosia crassifolia. The flavonoids Glabranine and 7-O-methyl-Glabranine presented 70% inhibition on the dengue virus at a concentration of 25 microM, while methyl-hildgardtol A, hildgardtol A and elongatine had no effect on viral growth. Our results show that Glabranine and 7-O-methyl-Glabranine isolated from Tephrosia s.p. exert a dose-dependent inhibitory effect in vitro on the dengue virus.
Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum teretifolium.[Pubmed:25903365]
Molecules. 2015 Apr 20;20(4):7143-55.
Ten flavonoid-related structures viz. heliteretifolin (1), isoxanthohumol (2), 2',4',6'-trihydroxy-3'-prenylchalcone (3), isoGlabranin (4), Glabranin (5), 7-methoxy-isoGlabranin (6), quercetin (7), 4'-methoxyquercetin (8), 4'-methoxykaempferol (9) and mosloflavone (10) were isolated from a H. teretifolium methanolic extract and identified. One of them (compound 1) is reported for the first time from a natural source, while compounds 6, 8-10 were isolated for the first time from the genus Helichrysum. The total extract of H. teretifolium showed potent antioxidant activity. When tested for total antioxidant capacity compound 3 possesses moderate biological activity compared to 2, which displayed some of the highest TEAC values (4529.01 +/- 2.44; 4170.66 +/- 6.72) microM TE/g, respectively. Compounds 7 and 8 demonstrated the highest inhibitory activities on Fe2+-induced lipid peroxidation (IC50 = 2.931; 6.449 microg/mL); tyrosinase (8.092; 27.573) and elastase (43.342; 86.548). Additionally, the total antioxidant capacities measured as FRAP (4816.31 +/- 7.42; 3584.17 +/- 0.54) microM AAE/g, and ORAC for hydroxyl radical (7.265 +/- 0.71; 6.779 +/- 3.40) x 106 and peroxyl radical (17.836 +/- 2.90; 12.545 +/- 5.07) x 103 microM TE/g were also observed for compounds 7 and 8, respectively. In conclusion, H. teretifolium total extract represents a rich source of bioactive constituents with potent antioxidant and moderate anti-tyrosinase and anti-elastase activities that can help to avert accumulation of free radicals in the body, and could therefore be good candidates for the prevention and/or treatment of skin-related conditions, such as aging. This is the first scientific report on the chemical and biological profile of H. teretifolium.
Antioxidant activity-guided separation of coumarins and lignan from Melicope glabra (Rutaceae).[Pubmed:23561082]
Food Chem. 2013 Aug 15;139(1-4):87-92.
The ethyl acetate and methanol bark extracts of Melicope glabra were evaluated for their antioxidant capacities by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity and beta-carotene bleaching/linoleic acid system. Both extracts exhibited strong inhibition against the DPPH radical (IC50 values of 24.81 and 13.01 mug ml(-1), respectively) and strong antioxidant activity in beta-carotene bleaching assay. Both samples were found to have high phenolic content with values of 39 and 44 mg GAE/g as indicated by Follin-Ciocalteau's reagent. Antioxidant TLC assay-guided isolation on the methanol extract led to the isolation of a new pyranocoumarin, Glabranin (1), umbelliferone (2), scopoletin (3) and sesamin (4), and their structures were determined by spectroscopy. Compounds (1-3) showed significant activities on DPPH free radical with the IC50 of 240.20, 810.02 and 413.19 mug ml(-1), respectively. However, in beta-carotene bleaching assay, sesamin (4) showed higher inhibitory activity (1 mg ml(-1), 95%) than Glabranin (1) (1 mg ml(-1), 74%), whilst umbelliferone (2) and scopoletin (3) were slightly pro-oxidant.
Dihydrostilbene derivatives from Glycyrrhiza glabra leaves.[Pubmed:16038558]
J Nat Prod. 2005 Jul;68(7):1099-102.
Four new dihydrostilbenes, alpha,alpha'-dihydro-3,5-dihydroxy-4'-acetoxy-5'-isopentenylstilbene (1), alpha,alpha'-dihydro-3,3',4'-trihydroxy-5-O-isopentenyl-6-isopentenylstilbene (2), alpha,alpha'-dihydro-3,5,3'-trihydroxy-4'-methoxystilbene (3), and alpha,alpha'-dihydro-3,3'-dihydroxy-5beta-d-O-glucopyranosyloxy-4'-methoxystilben e (4), together with seven known flavonoids, Glabranin isomer, naringenin, lupiwighteone, pinocembrin 7-O-glucoside, astragalin, isoquercitrin, vicenin II, and the inositol, pinitol, were isolated from the leaves of Glycyrrhiza glabra grown in Sicily. The structures of 1-4 were elucidated by spectroscopic methods.


