Antitumor Compound 1CAS# 420126-30-3 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 420126-30-3 | SDF | Download SDF |
| PubChem ID | 73427118 | Appearance | Powder |
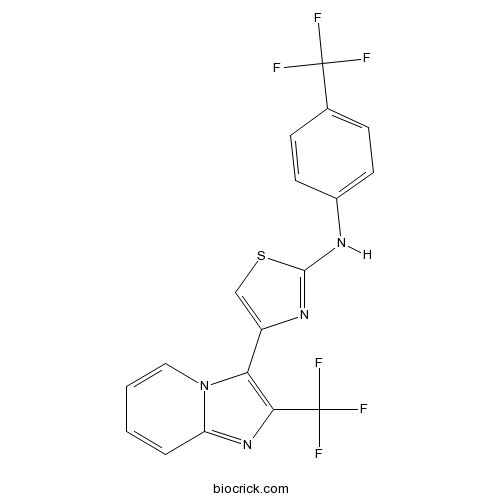
| Formula | C18H10F6N4S | M.Wt | 428.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 59 mg/mL (137.74 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[2-(trifluoromethyl)imidazo[1,2-a]pyridin-3-yl]-N-[4-(trifluoromethyl)phenyl]-1,3-thiazol-2-amine | ||
| SMILES | C1=CC2=NC(=C(N2C=C1)C3=CSC(=N3)NC4=CC=C(C=C4)C(F)(F)F)C(F)(F)F | ||
| Standard InChIKey | KYPPJHVEENIEGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H10F6N4S/c19-17(20,21)10-4-6-11(7-5-10)25-16-26-12(9-29-16)14-15(18(22,23)24)27-13-3-1-2-8-28(13)14/h1-9H,(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Antitumor Compound 1 Dilution Calculator

Antitumor Compound 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3345 mL | 11.6727 mL | 23.3454 mL | 46.6908 mL | 58.3635 mL |
| 5 mM | 0.4669 mL | 2.3345 mL | 4.6691 mL | 9.3382 mL | 11.6727 mL |
| 10 mM | 0.2335 mL | 1.1673 mL | 2.3345 mL | 4.6691 mL | 5.8363 mL |
| 50 mM | 0.0467 mL | 0.2335 mL | 0.4669 mL | 0.9338 mL | 1.1673 mL |
| 100 mM | 0.0233 mL | 0.1167 mL | 0.2335 mL | 0.4669 mL | 0.5836 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Antitumor Compound 1 is a potent compound which comprises a new imidazopyridine having excellent antitumor activity as an active ingredient. Reference: Polymer-linked imidazopyridines useful as tumor-targeting cytotoxic agents By Kasuya, Hiroshi; Miyazaki, Hideki; Hayakawa, Ichio; Kanno, Yuichi; Watanabe, Kazuyoshi From Jpn. Kokai Tokkyo Koho (2004), JP 2004002826 A 20040108. Pharmaceuticals containing imidazopyridines for prophylactic and therapeutic treatment of tumor By Hayakawa, Ichio; Kanno, Yuichi; Azuma, Toshiki; Furukawa, Hidehiko; Naruto, Shunji; Kurakata, Shinichi From Jpn. Kokai Tokkyo Koho (2003), JP 2003313126A 20031106. Preparation of imidazopyridine derivatives as antitumor agents By Hayakawa, Ichiro; Sugano, Yuichi; Agatsuma, Toshinori; Furukawa, Hidehiko; Kurakata, Shinichi; Naruto, Shunji From PCT Int. Appl. (2002), WO 2002034748 A1 20020502.
- Dihydropashanone
Catalog No.:BCN4635
CAS No.:41997-41-5
- 5-Chloro-1,10-phenanthroline
Catalog No.:BCC3713
CAS No.:4199-89-7
- (S)-(-)-Propranolol hydrochloride
Catalog No.:BCC6809
CAS No.:4199-10-4
- Glabranin
Catalog No.:BCN5480
CAS No.:41983-91-9
- Pinocembrin chalcone
Catalog No.:BCN7223
CAS No.:4197-97-1
- MLR 1023
Catalog No.:BCC6232
CAS No.:41964-07-2
- N1-Methyl-4-nitrobenzene-1,2-diamine
Catalog No.:BCC9070
CAS No.:41939-61-1
- Trilobatin
Catalog No.:BCN5479
CAS No.:4192-90-9
- Isopropyl 4-Hydroxybenzoate
Catalog No.:BCN8409
CAS No.:4191-73-5
- Nitrocefin
Catalog No.:BCC6544
CAS No.:41906-86-9
- PYR-41
Catalog No.:BCC4470
CAS No.:418805-02-4
- 2,3-DCPE hydrochloride
Catalog No.:BCC2384
CAS No.:418788-90-6
- Fenofibric acid
Catalog No.:BCC8982
CAS No.:42017-89-0
- Estriol 17-sulfate
Catalog No.:BCN2237
CAS No.:42028-21-7
- Clonidine HCl
Catalog No.:BCC4325
CAS No.:4205-91-8
- Mezlocillin Sodium
Catalog No.:BCC4678
CAS No.:42057-22-7
- Atractylenolide III acetate
Catalog No.:BCC9147
CAS No.:
- d[Leu4,Lys8]-VP
Catalog No.:BCC5981
CAS No.:42061-33-6
- 2 CTC
Catalog No.:BCC2571
CAS No.:42074-68-0
- Lucialdehyde A
Catalog No.:BCN2449
CAS No.:420781-84-6
- AK-7
Catalog No.:BCC5426
CAS No.:420831-40-9
- H2L 5765834
Catalog No.:BCC6311
CAS No.:420841-84-5
- Tetradehydropodophyllotoxin
Catalog No.:BCN8395
CAS No.:42123-27-3
- N,N-Bis(2-chloroethyl)-p-toluenesulphonamide
Catalog No.:BCC9060
CAS No.:42137-88-2
In vitro and in vivo antitumor activity of a novel carbonyl ruthenium compound, the ct-[RuCl(CO)(dppb)(bipy)]PF-6[dppb=1,4-bis(diphenylphosphine)butane and bipy=2,2'-bipyridine].[Pubmed:27613330]
J Inorg Biochem. 2016 Nov;164:42-48.
This study performed in vitro and in vivo biological assays of the ruthenium (II) compound ct-[RuCl(CO)(dppb)(bipy)]PF6 (where, dppb=1,4-bis(diphenylphosphine)butane and bipy=2,2'-bipyridine). The cytotoxic activity of this compound was evaluated against different tumor cell lines (HeLa, human cervical adenocarcinoma; MCF7, human breast adenocarcinoma; MO59J, human glioblastoma; HepG2, hepatocellular carcinoma and B16F10, murine melanoma) and healthy cell line (V79, Chinese hamster lung fibroblasts), by XTT (sodium 2,3'-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-3,4-tetrazol ium-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate) method. A syngeneic murine melanoma tumor model (B16F10) was used to evaluate its antitumor activity. Additionally, experiments were performed to assess the interactions with ctDNA (calf thymus DNA) and BSA (bovine serum albumin). The results showed that ct-[RuCl(CO)(dppb)(bipy)]PF6 was cytotoxic against all tumor cell lines tested. Furthermore, the compound was more effective against tumor cells compared to the normal cell line, indicating selectivity, especially in B16F10 cells. Significant tumor growth reduction was observed in animals treated with the compound compared to the untreated control. Histopathological analysis of tumor tissue revealed a significant reduction of mitosis in animals treated with the compound compared to the untreated control. In the ctDNA and BSA interaction experiments, the compound in study showed weak interactions with ctDNA and hydrophobic interactions with BSA. The ruthenium compound investigated showed promising results in in vitro and in vivo biological assays.
Antitumor effects of novel compound, guttiferone K, on colon cancer by p21Waf1/Cip1-mediated G(0) /G(1) cell cycle arrest and apoptosis.[Pubmed:22733377]
Int J Cancer. 2013 Feb 1;132(3):707-16.
Low selectivity is one of the major problems of currently used anticancer drugs, therefore, there is a high demand for novel, selective antitumor agents. In this study, the anticancer effects and mechanisms of guttiferone K (GUTK), a novel polyprenylated acylphloroglucinol derivative isolated from Garcinia cowa Roxb., were examined for its development as a novel drug targeting colon cancer. GUTK concentration- and time-dependently reduced the viability of human colon cancer HT-29 cells (IC(50) value 5.39 +/- 0.22 muM) without affecting the viability of normal human colon epithelial CCD 841 CoN cells and induced G(0) /G(1) cell cycle arrest in HT-29 cells by down-regulating cyclins D1, D3 and cyclin-dependent kinases 4 and 6, while selectively restoring p21Waf1/Cip1 and p27Kip1 to levels comparable to those observed in normal colon cells, without affecting their levels in normal cells. GUTK (10.0 muM) induced cleavage of PARP, caspases-3, -8 and -9 and chromatin condensation to stimulate caspase-3-mediated apoptosis. The addition of a JNK inhibitor, SP600125, partially reversed GUTK-induced caspase-3 activity, indicating the possible involvement of JNK in GUTK-induced apoptosis. Furthermore, GUTK (10 mg/kg, i.p.) significantly decreased the tumor volume in a syngeneic colon tumor model when used alone or in combination with 5-fluorouracil without toxicity to the mice. Immunohistochemical staining of the tumor sections revealed a mechanism involving an increase in cleaved caspase-3 and a decrease in cell proliferation marker Ki-67. Our results support GUTK as a promising novel, potent and selective antitumor drug candidate for colon cancer.
A novel, broad-spectrum antitumor compound containing the 1-hydroxycyclohexa-2,5-dien-4-one group: the disclosure of a new antitumor pharmacophore in protoapigenone 1.[Pubmed:21515048]
Bioorg Med Chem Lett. 2011 Jun 1;21(11):3427-30.
The synthesis of a new compound 9 containing the 4-hydroxy-2,5-cyclohexadien-1-one system, a key elements toward elucidation of the protoapigenone 1 antitumor pharmacophore, was described. The compound showed potent in vitro antitumor potency with low micromolar IC(50)'s against breast, ovarian, prostate, liver, pancreas, and blood cancer cell lines tested and could inhibit tumor growth in vivo but no significant impairment of hematopoiesis or immune function was observed. The minimum structural pharmacophore of 1 has now been refined.
The antidiabetic compound 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione, isolated from Averrhoa carambola L., demonstrates significant antitumor potential against human breast cancer cells.[Pubmed:26203774]
Oncotarget. 2015 Sep 15;6(27):24304-19.
2-Dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (DMDD) is a cyclohexanedione found in the roots of Averrhoa carambola L., commonly known as starfruit. Researchers have shown that DMDD has significant therapeutic potential for the treatment of diabetes; however, the effects of DMDD on human cancers have never been reported. We investigated the cytotoxic effects of DMDD against human breast, lung and bone cancer cells in vitro and further examined the molecular mechanisms of DMDD-induced apoptosis in human breast cancer cells. DMDD suppressed the growth of breast carcinoma cells, but not normal mammary epithelial cells, via induction of G1 phase cell cycle arrest, oxidative stress and apoptosis. DMDD increased the level of intracellular reactive oxygen species (ROS) and DMDD-induced ROS generation was found to be associated with the mitochondrial activity. The cytotoxicity that was induced by DMDD was attenuated by co-treatment with the antioxidant N-acetyl-L-cysteine (NAC). DMDD-induced cell apoptosis involved the activation of both the intrinsic mitochondrial pathway and the extrinsic receptor pathway. In addition, DMDD inhibited the canonical NF-kappaB signaling pathway at all steps, including TNF-alpha production, phosphorylation of NF-kappaB p65 and IkappaBalpha, as well as TNF-alpha activated NF-kappaB p65 nuclear translocation.Collectively, our studies indicate that DMDD has significant potential as a safe and efficient therapeutic agent for the treatment of breast cancer.


