ACBCCAS# 22264-50-2 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 22264-50-2 | SDF | Download SDF |
| PubChem ID | 89643 | Appearance | Powder |
| Formula | C5H9NO2 | M.Wt | 115.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
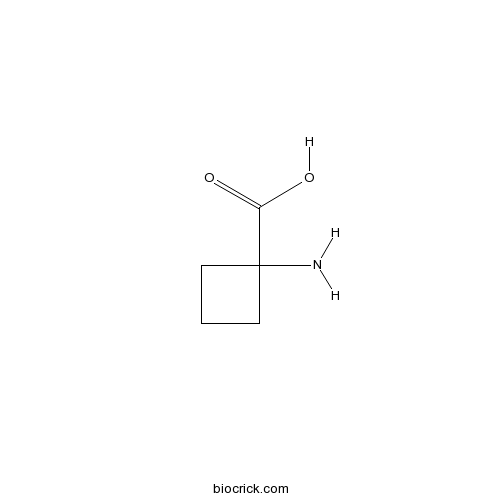
| Chemical Name | 1-aminocyclobutane-1-carboxylic acid | ||
| SMILES | C1CC(C1)(C(=O)O)N | ||
| Standard InChIKey | FVTVMQPGKVHSEY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H9NO2/c6-5(4(7)8)2-1-3-5/h1-3,6H2,(H,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NMDA receptor partial agonist, acting at the glycine site of GluN1 (formally NR1). |

ACBC Dilution Calculator

ACBC Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6858 mL | 43.4292 mL | 86.8583 mL | 173.7167 mL | 217.1458 mL |
| 5 mM | 1.7372 mL | 8.6858 mL | 17.3717 mL | 34.7433 mL | 43.4292 mL |
| 10 mM | 0.8686 mL | 4.3429 mL | 8.6858 mL | 17.3717 mL | 21.7146 mL |
| 50 mM | 0.1737 mL | 0.8686 mL | 1.7372 mL | 3.4743 mL | 4.3429 mL |
| 100 mM | 0.0869 mL | 0.4343 mL | 0.8686 mL | 1.7372 mL | 2.1715 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Antidesmone
Catalog No.:BCN5058
CAS No.:222629-77-8
- Bromocriptine mesylate
Catalog No.:BCC6642
CAS No.:22260-51-1
- Tempol
Catalog No.:BCC4862
CAS No.:2226-96-2
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- Loganic acid
Catalog No.:BCN5057
CAS No.:22255-40-9
- Lucidin 3-O-glucoside
Catalog No.:BCN8249
CAS No.:22255-29-4
- Trimethoxystilbene
Catalog No.:BCN6762
CAS No.:22255-22-7
- Guaijaverin
Catalog No.:BCN5056
CAS No.:22255-13-6
- alpha-Amyrin palmitate
Catalog No.:BCN5055
CAS No.:22255-10-3
- Methyl 6-hydroxyangolensate
Catalog No.:BCN5054
CAS No.:22255-07-8
- Ipratropium Bromide
Catalog No.:BCC3795
CAS No.:22254-24-6
- 7-Amino-3-methyl-3-cephem-4-carboxylic acid
Catalog No.:BCC8776
CAS No.:22252-43-3
- Chysin A
Catalog No.:BCN2020
CAS No.:22269-11-0
- SZL P1-41
Catalog No.:BCC8004
CAS No.:222716-34-9
- Noladin ether
Catalog No.:BCC5756
CAS No.:222723-55-9
- Finasteride acetate
Catalog No.:BCC4204
CAS No.:222989-99-3
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Evodol
Catalog No.:BCN5059
CAS No.:22318-10-1
- Platycodigenin
Catalog No.:BCN3183
CAS No.:22327-82-8
- Methyl ferulate
Catalog No.:BCN4023
CAS No.:22329-76-6
- 9-Hydroxy-alpha-lapachone
Catalog No.:BCN5060
CAS No.:22333-58-0
- Grandiflorenic acid
Catalog No.:BCN4670
CAS No.:22338-67-6
Positron Emission Tomography (PET) with 1-Aminocyclobutane-1-[(11)C]carboxylic Acid (1-[(11)C]-ACBC) for Detecting Recurrent Brain Tumors.[Pubmed:14516591]
Clin Positron Imaging. 1998 Jun;1(3):165-173.
This study was done to determine whether 1-[(11)C]ACBC PET has any advantages over 2-[(18)F]FDG PET, CT, or MRI in detecting recurrent brain tumors, and whether quantitative 1-[(11)C]ACBC PET information improves the accuracy of "visual" image interpretation.Twenty patients with recurrent brain tumor underwent dynamic PET. Images were analyzed by visual interpretation; in addition, standardized uptake values (SUVs) and Patlak values (k(1)*k(3)/k) were evaluated.1-[(11)C]ACBC identified 19/20 recurrent brain tumors, [18F]FDG 13/19, MRI 13/19, and CT 8/16. Based on SUVs, the average tumor-to-contralateral gray matter ratio of 1-[(11)C]ACBC was 5.0 and 0.5 for 2-[(18)F]FDG. Mean Patlak values of 1-[(11)C]ACBC were 0.044 +/- 0.047 for high and 0.034 +/- 0.026 for low grade tumors. However, visual interpretation was effective without quantitative PET data.1-[(11)C]ACBC, accurately detects recurrent tumors for selecting biopsy sites and treatment planning.
Generating Individual Patient Preferences for the Treatment of Osteoarthritis Using Adaptive Choice-Based Conjoint (ACBC) Analysis.[Pubmed:28255898]
Rheumatol Ther. 2017 Jun;4(1):167-182.
INTRODUCTION: To explore how adaptive choice-based conjoint (ACBC) analysis could contribute to shared decision-making in the treatment of individual patients with osteoarthritis (OA). METHODS: In-depth case study of three individuals randomly selected from patients with OA participating in an ACBC analysis exercise. Eleven members of a research users' group participated in developing an ACBC task on medication preferences for OA. Individual medication priorities are illustrated by the detailed analysis of ACBC output from three randomly selected patients from the main sample. RESULTS: The case study analysis illustrates individual preferences. Participant 1's priority was avoidance of the four high-risk side effects of medication, which accounted for 90% of the importance of all attributes, while the remaining attributes (expected benefit; way of taking medication; frequency; availability) accounted only for 10% of the total influence. Participant 3 was similar to participant 1 but would accept a high risk of one of the side effects if the medication were available by prescription. In contrast, participant 2's priority was the avoidance of Internet purchase of medication; this attribute (availability) accounted for 52% of the importance of all attributes. CONCLUSIONS: Individual patients have preferences that likely lead to different medication choices. ACBC has the potential as a tool for physicians to identify individual patient preferences as a practical basis for concordant prescribing for OA in clinical practice. Future research needs to establish whether accurate knowledge of individual patient preferences for treatment attributes and levels translates into concordant behavior in clinical practice.
Differential Effects of D-Cycloserine and ACBC at NMDA Receptors in the Rat Entorhinal Cortex Are Related to Efficacy at the Co-Agonist Binding Site.[Pubmed:26193112]
PLoS One. 2015 Jul 20;10(7):e0133548.
Partial agonists at the NMDA receptor co-agonist binding site may have potential therapeutic efficacy in a number of cognitive and neurological conditions. The entorhinal cortex is a key brain area in spatial memory and cognitive processing. At synapses in the entorhinal cortex, NMDA receptors not only mediate postsynaptic excitation but are expressed in presynaptic terminals where they tonically facilitate glutamate release. In a previous study we showed that the co-agonist binding site of the presynaptic NMDA receptor is endogenously and tonically activated by D-serine released from astrocytes. In this study we determined the effects of two co-agonist site partial agonists on both presynaptic and postsynaptic NMDA receptors in layer II of the entorhinal cortex. The high efficacy partial agonist, D-cycloserine, decreased the decay time of postsynaptic NMDA receptor mediated currents evoked by electrical stimulation, but had no effect on amplitude or other kinetic parameters. In contrast, a lower efficacy partial agonist, 1-aminocyclobutane-1-carboxylic acid, decreased decay time to a greater extent than D-cycloserine, and also reduced the peak amplitude of the evoked NMDA receptor mediated postsynaptic responses. Presynaptic NMDA receptors, (monitored indirectly by effects on the frequency of AMPA receptor mediated spontaneous excitatory currents) were unaffected by D-cycloserine, but were reduced in effectiveness by 1-aminocyclobutane-1-carboxylic acid. We discuss these results in the context of the effect of endogenous regulation of the NMDA receptor co-agonist site on receptor gating and the potential therapeutic implications for cognitive disorders.
Contribution of nucleus accumbens core (AcbC) to behavior control during a learned resting period: introduction of a novel task and lesion experiments.[Pubmed:24776793]
PLoS One. 2014 Apr 28;9(4):e95941.
In recent years, the study of resting state neural activity has received much attention. To better understand the roles of different brain regions in the regulation of behavioral activity in an arousing or a resting period, we developed a novel behavioral paradigm (8-arm food-foraging task; 8-arm FFT) using the radial 8-arm maze and examined how ACBC lesions affect behavioral execution and learning. Repetitive training on the 8-arm FFT facilitated motivation of normal rats to run quickly to the arm tips and to the center platform before the last-reward collection. Importantly, just after this point and before confirmation of no reward at the next arm traverse, locomotor activity decreased. This indicates that well-trained rats can predict the absence of the reward at the end of food seeking and then start another behavior, namely planned resting. Lesions of the ACBC after training selectively impaired this reduction of locomotor activity after the last-reward collection without changing activity levels before the last-reward collection. Analysis of arm-selection patterns in the lesioned animals suggests little influence of the lesion in the ability to predict the reward absence. ACBC lesions did not change exploratory locomotor activity in an open-field test in which there were no rewards. This suggests that the ACBC controls the activity level of planned resting behavior shaped by the 8-arm FFT. Rats receiving training after ACBC lesioning showed a reduction in motivation for reward seeking. Thus, the ACBC also plays important roles not only in controlling the activity level after the last-reward collection but also in motivational learning for setting the activity level of reward-seeking behavior.
Mechanism of partial agonist action at the NR1 subunit of NMDA receptors.[Pubmed:15996549]
Neuron. 2005 Jul 7;47(1):71-84.
Partial agonists produce submaximal activation of ligand-gated ion channels. To address the question of partial agonist action at the NR1 subunit of the NMDA receptor, we performed crystallographic and electrophysiological studies with 1-aminocyclopropane-1-carboxylic acid (ACPC), 1-aminocyclobutane-1-carboxylic acid (ACBC), and 1-aminocyclopentane-1-carboxylic acid (cycloleucine), three compounds with incrementally larger carbocyclic rings. Whereas ACPC and ACBC partially activate the NMDA receptor by 80% and 42%, respectively, their cocrystal structures of the NR1 ligand binding core show the same degree of domain closure as found in the complex with glycine, a full agonist, illustrating that the NR1 subunit provides a new paradigm for partial agonist action that is distinct from that of the evolutionarily related GluR2, AMPA-sensitive receptor. Cycloleucine behaves as an antagonist and stabilizes an open-cleft conformation. The NR1-cycloleucine complex forms a dimer that is similar to the GluR2 dimer, thereby suggesting a conserved mode of subunit-subunit interaction in AMPA and NMDA receptors.
Neuropharmacological characterization of 1-aminocyclopropane-1-carboxylate and 1-aminocyclobutane-1-carboxylate, ligands of the N-methyl-D-aspartate-associated glycine receptor.[Pubmed:2158004]
Neuropharmacology. 1990 Mar;29(3):305-9.
Following intravenous administration, 1-aminocyclobutane-1-carboxylate (ACBC, 100 mg/kg), a N-methyl-D-aspartate (NMDA)-associated glycine receptor antagonist, was eliminated with a T1/2 of 5 min in mouse brain and 4 min in rat cerebrospinal fluid (CSF). 1-Aminocyclopropane-1-carboxylate (ACC), a NMDA-associated glycine receptor agonist, was found to have a T1/2 of less than 5 min in mouse brain. ACC and ACBC did not alter basal cerebellar cGMP. Glycine and D-serine increased cGMP, and 1-hydroxy-3-aminopyrrolidone-2 (HA-966), a glycine antagonist, reversed the D-serine-induced increases in cGMP. In contrast, ACBC did not reverse the D-serine-induced increases in cGMP. These data suggest that despite their brain bioavailability and marked potency at the glycine receptor in vitro, ACC and ACBC are rapidly inactivated and thus have limited in vivo utility.


