FlumazenilBenzodiazepine antagonist CAS# 78755-81-4 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 78755-81-4 | SDF | Download SDF |
| PubChem ID | 3373 | Appearance | Powder |
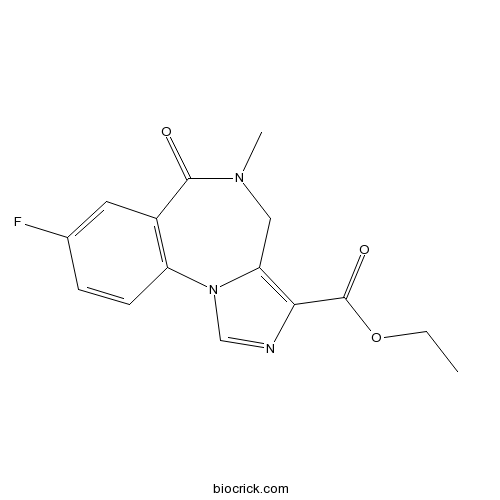
| Formula | C15H14FN3O3 | M.Wt | 303.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ro 15-1788 | ||
| Solubility | DMSO : 20 mg/mL (65.94 mM; Need ultrasonic) | ||
| Chemical Name | ethyl 8-fluoro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate | ||
| SMILES | CCOC(=O)C1=C2CN(C(=O)C3=C(N2C=N1)C=CC(=C3)F)C | ||
| Standard InChIKey | OFBIFZUFASYYRE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benzodiazepine antagonist, non-selective for α1, α2, α3 or α5-containing GABAA receptors. Centrally active upon systemic administration in vivo. |

Flumazenil Dilution Calculator

Flumazenil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2972 mL | 16.4859 mL | 32.9717 mL | 65.9435 mL | 82.4294 mL |
| 5 mM | 0.6594 mL | 3.2972 mL | 6.5943 mL | 13.1887 mL | 16.4859 mL |
| 10 mM | 0.3297 mL | 1.6486 mL | 3.2972 mL | 6.5943 mL | 8.2429 mL |
| 50 mM | 0.0659 mL | 0.3297 mL | 0.6594 mL | 1.3189 mL | 1.6486 mL |
| 100 mM | 0.033 mL | 0.1649 mL | 0.3297 mL | 0.6594 mL | 0.8243 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Benzodiazepine antagonist, non-selective for α1, α2, α3 or α5-containing GABAA receptors. Centrally active upon systemic administration in vivo.
- Shizukanolide C
Catalog No.:BCN6570
CAS No.:78749-47-0
- D-AP4
Catalog No.:BCC6549
CAS No.:78739-01-2
- Ozagrel HCl
Catalog No.:BCC4926
CAS No.:78712-43-3
- Plantagoside
Catalog No.:BCN8077
CAS No.:78708-33-5
- 4,4'-Biphenyldicarboxylic acid
Catalog No.:BCC8655
CAS No.:787-70-2
- Z-D-Asp-OH
Catalog No.:BCC2786
CAS No.:78663-07-7
- Dehydroandrographolidesuccinate
Catalog No.:BCN8359
CAS No.:786593-06-4
- Terbinafine HCl
Catalog No.:BCC4863
CAS No.:78628-80-5
- Amorolfine HCl
Catalog No.:BCC4889
CAS No.:78613-38-4
- JDTic 2HCl
Catalog No.:BCC1671
CAS No.:785835-79-2
- Cycloastragenol
Catalog No.:BCN8483
CAS No.:78574-94-4
- FG 7142
Catalog No.:BCC6694
CAS No.:78538-74-6
- TC 1698 dihydrochloride
Catalog No.:BCC7394
CAS No.:787587-06-8
- Deapi-platycodin D
Catalog No.:BCN2614
CAS No.:78763-58-3
- Calcifediol-D6
Catalog No.:BCC4075
CAS No.:78782-98-6
- Calcitriol D6
Catalog No.:BCC1447
CAS No.:78782-99-7
- Zeylenol
Catalog No.:BCC8267
CAS No.:78804-17-8
- 4-Benzyloxycarbonyl-2-piperazinone
Catalog No.:BCC8699
CAS No.:78818-15-2
- Epibrassinolide
Catalog No.:BCC5479
CAS No.:78821-43-9
- Garcinol
Catalog No.:BCC5623
CAS No.:78824-30-3
- Demethylasterriquinone B1
Catalog No.:BCC7189
CAS No.:78860-34-1
- 1-chloro-6-(5-(prop-1-ynyl)thiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1352
CAS No.:78876-52-5
- 1-chloro-6-(5-ethynylthiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1351
CAS No.:78876-53-6
- 6-Thio-dG
Catalog No.:BCC6507
CAS No.:789-61-7
GABAA receptor subtypes in the mouse brain: Regional mapping and diazepam receptor occupancy by in vivo [(18)F]flumazenil PET.[Pubmed:28192273]
Neuroimage. 2017 Apr 15;150:279-291.
Classical benzodiazepines, which are widely used as sedatives, anxiolytics and anticonvulsants, exert their therapeutic effects through interactions with heteropentameric GABAA receptors composed of two alpha, two beta and one gamma2 subunit. Their high affinity binding site is located at the interface between the gamma2 and the adjacent alpha subunit. The alpha-subunit gene family consists of six members and receptors can be homomeric or mixed with respect to the alpha-subunits. Previous work has suggested that benzodiazepine binding site ligands with selectivity for individual GABAA receptor subtypes, as defined by the benzodiazepine-binding alpha subunit, may have fewer side effects and may even be effective in diseases, such as schizophrenia, autism or chronic pain, that do not respond well to classical benzodiazepines. The distributions of the individual alpha subunits across the CNS have been extensively characterized. However, as GABAA receptors may contain two different alpha subunits, the distribution of the subunits does not necessarily reflect the distribution of receptor subtypes with respect to benzodiazepine pharmacology. In the present study, we have used in vivo [(18)F]Flumazenil PET and in vitro [(3)H]Flumazenil autoradiography in combination with GABAA receptor point-mutated mice to characterize the distribution of the two most prevalent GABAA receptor subtypes (alpha1 and alpha2) throughout the mouse brain. The results were in agreement with published in vitro data. High levels of alpha2-containing receptors were found in brain regions of the neuronal network of anxiety. The alpha1/alpha2 subunit combinations were predictable from the individual subunit levels. In additional experiments, we explored in vivo [(18)F]Flumazenil PET to determine the degree of receptor occupancy at GABAA receptor subtypes following oral administration of diazepam. The dose to occupy 50% of sensitive receptors, independent of the receptor subtype(s), was 1-2mg/kg, in agreement with published data from ex vivo studies with wild type mice. In conclusion, we have resolved the quantitative distribution of alpha1- and alpha2-containing homomeric and mixed GABAA receptors in vivo at the millimeter scale and demonstrate that the regional drug receptor occupancy in vivo at these GABAA receptor subtypes can be determined by [(18)F]Flumazenil PET. Such information should be valuable for drug development programs aiming for subtype-selective benzodiazepine site ligands for new therapeutic indications.
Contribution of neuroinflammation to changes in [(11)C]flumazenil binding in the rat brain: Evaluation of the inflamed pons as reference tissue.[Pubmed:28364664]
Nucl Med Biol. 2017 Jun;49:50-56.
INTRODUCTION: [(11)C]Flumazenil is a well-known PET tracer for GABAA receptors and is mainly used as an imaging biomarker for neuronal loss. Recently, GABAA receptors on immune cells have been investigated as target for modulation of inflammation. Since neuronal loss is often accompanied by neuroinflammation, PET imaging with [(11)C]Flumazenil is potentially affected by infiltrating immune cells. This may also compromise the validity of using the pons as reference tissue in quantitative pharmacokinetic analysis. This study aims to evaluate whether inflammatory processes in the brain can influence [(11)C]Flumazenil uptake and affect the outcome of pharmacokinetic modeling when the pons is used as reference tissue. METHODS: The herpes simplex encephalitis (HSE) rat model is known to cause neuroinflammation in the brainstem. Dynamic [(11)C]Flumazenil PET scans of 60-min, accompanied by arterial blood sampling and metabolite analysis, were acquired at day 6-7days post-infection of male Wistar rats (HSE, n=5 and control, n=6). Additionally, the GABAA receptor was saturated by injection of unlabeled Flumazenil prior to the tracer injection in 4 rats per group. PET data were analyzed by pharmacokinetic modeling. RESULTS: No statistically significant differences were found in the volume of distribution (VT) or non-displaceable binding potential (BPND) between control and HSE rats in any of the brain regions. Pre-saturation with unlabeled Flumazenil resulted in a statistically significant reduction in [(11)C]Flumazenil VT in all brain regions. The BPND obtained from SRTM exhibited a good correlation to DVR - 1 values from the two-tissue compartment model, coupled with some level of underestimation. CONCLUSION: Reliable quantification of [(11)C]Flumazenil binding in rats can be obtained by pharmacokinetic analysis using the pons as a pseudo-reference tissue even in the presence of strong acute neuroinflammation.
Short-term treatment with flumazenil restores long-term object memory in a mouse model of Down syndrome.[Pubmed:28215510]
Neurobiol Learn Mem. 2017 Apr;140:11-16.
Down syndrome (DS) is a common genetic cause of intellectual disability yet no pro-cognitive drug therapies are approved for human use. Mechanistic studies in a mouse model of DS (Ts65Dn mice) demonstrate that impaired cognitive function is due to excessive neuronal inhibitory tone. These deficits are normalized by chronic, short-term low doses of GABAA receptor (GABAAR) antagonists in adult animals, but none of the compounds investigated are approved for human use. We explored the therapeutic potential of Flumazenil (FLUM), a GABAAR antagonist working at the benzodiazepine binding site that has FDA approval. Long-term memory was assessed by the Novel Object Recognition (NOR) testing in Ts65Dn mice after acute or short-term chronic treatment with FLUM. Short-term, low, chronic dose regimens of FLUM elicit long-lasting (>1week) normalization of cognitive function in both young and aged mice. FLUM at low dosages produces long lasting cognitive improvements and has the potential of fulfilling an unmet therapeutic need in DS.
Pharmacokinetic modeling of [(11)C]flumazenil kinetics in the rat brain.[Pubmed:28229437]
EJNMMI Res. 2017 Dec;7(1):17.
BACKGROUND: Preferred models for the pharmacokinetic analysis of [(11)C]Flumazenil human studies have been previously established. However, direct translation of these models and settings to animal studies might be sub-optimal. Therefore, this study evaluates pharmacokinetic models for the quantification of [(11)C]Flumazenil binding in the rat brain. Dynamic (60 min) [(11)C]Flumazenil brain PET scans were performed in two groups of male Wistar rats (tracer dose (TD), n = 10 and pre-saturated (PS), n = 2). Time-activity curves from five regions were analyzed, including the pons (pseudo-reference region). Distribution volume (VT) was calculated using one- and two-tissue compartment models (1TCM and 2TCM) and spectral analysis (SA). Binding potential (BPND) was determined from full and simplified reference tissue models with one or two compartments for the reference tissue (FRTM, SRTM, and SRTM-2C). Model preference was determined by Akaike information criterion (AIC), while parameter agreement was assessed by linear regression, repeated measurements ANOVA and Bland-Altman plots. RESULTS: 1TCM and 2TCM fits of regions with high specific binding showed similar AIC, a preference for the 1TCM, and good VT agreement (0.1% difference). In contrast, the 2TCM was markedly preferred and necessary for fitting low specific-binding regions, where a worse VT agreement (17.6% difference) and significant VT differences between the models (p < 0.005) were seen. The PS group displayed results similar to those of low specific-binding regions. All reference models (FRTM, SRTM, and SRTM-2C) resulted in at least 13% underestimation of BPND. CONCLUSIONS: Although the 1TCM was sufficient for the quantification of high specific-binding regions, the 2TCM was found to be the most adequate for the quantification of [(11)C]Flumazenil in the rat brain based on (1) higher fit quality, (2) lower AIC values, and (3) ability to provide reliable fits for all regions. Reference models resulted in negatively biased BPND and were affected by specific binding in the pons of the rat.
Regional differences in the inhibition of mouse in vivo [3H]Ro 15-1788 binding reflect selectivity for alpha 1 versus alpha 2 and alpha 3 subunit-containing GABAA receptors.[Pubmed:10063485]
Neuropsychopharmacology. 1999 Mar;20(3):255-62.
The benzodiazepines flunitrazepam, diazepam, and Ro 15-1788 and the beta-carboline DMCM bind with equivalent affinity to the benzodiazepine binding site of GABAA receptors containing different alpha subunits (i.e., alpha 1, alpha 2, alpha 3, or alpha 5); whereas, the triazolopyridazine CL 218,872 and imidazopyridine zolpidem have higher affinity for alpha 1 subunit-containing GABAA receptors. In the present study, the in vivo binding of [3H]Ro 15-1788 in mouse cerebellum and spinal cord was used to establish the occupancy of the benzodiazepine binding site of GABAA receptors containing primarily alpha 1 and alpha 2/alpha 3 subunits, respectively. Thus, the nonselective compounds flunitrazepam, diazepam, and DMCM all produced a similar inhibition of binding in cerebellum and spinal cord (respective ID50 values of 0.2 to 0.3 mg/kg, 2 mg/kg, and 10 mg/kg i.p.); whereas, the alpha 1 selective compounds CL 218,872 and zolpidem were more potent at inhibiting [3H]Ro 15-1788 binding in the cerebellum (ID50 values 4.5 mg/kg and 10 mg/kg i.p.) compared to the spinal cord (ID50 values 12 mg/kg and > 30 mg/kg i.p.). Thus, the reduction of in vivo f[3H]Ro 15-1788 binding in tissues containing alpha 1 and alpha 2/alpha 3 receptor populations reflects the in vitro affinities of subtype selective compounds and should help to interpret the behavioral profile of such compounds.
New insights into the mechanism of action of hypnotics.[Pubmed:10667451]
J Psychopharmacol. 1999;13(4 Suppl 1):S11-20.
Between 1987 and 1989, the different protein subunits that make up the receptor for the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) were identified. These make up the alpha, beta, gamma and delta families, for each of which exist several subtypes. This receptor is the molecular target of modern hypnotic drugs (i.e. benzodiazepines, zopiclone, zolpidem and zaleplon). In the 10 years that have followed this milestone, significant progress has been made in exploring the molecular mechanisms of hypnotic drug action. Receptor subtype specificity of hypnotics has been explained in terms of differential affinity for receptors containing different alpha subunits, which are expressed in different brain regions. Zolpidem and zaleplon bind preferentially to alpha1-containing receptors, whereas benzodiazepines and zopiclone are aspecific. Different sets of subunits are encoded in contiguous 'cassettes' on the genome, and the transcription of each set appears to be regulated coherently. The predominant GABA(A) receptor composition found in the brain is alpha1beta2gamma2, which are all encoded on human chromosome 5. Targeted gene disruption has provided clues to the physiological functions served by GABA(A) receptors containing different subunits. Receptors containing gamma2 appear to have a vital role in maintaining appropriate central inhibition, beta3-containing receptors may also be important determinants of excitability in certain brain regions, whereas a clear role for alpha5-, alpha6- and gamma3-containing receptors has not yet been established by these techniques. Site-directed mutagenesis has indicated that benzodiazepines bind to a cleft on the GABA(A) receptor surface at the interface between the alpha and gamma subunits. Other drugs (Flumazenil, zopiclone, zolpidem) also bind to the a subunit, but interact with amino acids in different binding domains to the benzodiazepines. The molecular mechanism of hypnotic dependence has been explored, and seems to involve downregulation of transcription of the normally prevalent alpha1, beta2 and gamma2 subunits, and the reciprocal upregulation of the expression of rarer subunits. Chronic treatment with hypnotic drugs that may have less dependence potential, such as zopiclone and zolpidem, appears to produce more limited change in GABA(A) receptor subunit expression. These ideas will be important both for designing new hypnotic drugs with a better safety/efficacy profile, and for evaluating more appropriate ways of using the drugs available today.
Electrophysiological studies on the specific benzodiazepine antagonist Ro 15-1788.[Pubmed:7196507]
Naunyn Schmiedebergs Arch Pharmacol. 1981 Jul;316(4):317-25.
This is an electrophysiological study in cats and rats of the imidazobenzodiazepinone derivative, Ro 15-1788, the first representative of specific benzodiazepine antagonists. (1) In unanaesthetized spinal cats, 1-10 mg kg-1 Ro 15-1788 i.v. did not affect segmental dorsal root potentials (DRPs), polysynaptic ventral root reflexes (VRRs), Renshaw cell responses to antidromic ventral root volleys and spontaneous gamma-motoneurone activity. However, at 1 mg kg-1 i.v., it antagonized the enhancement of DRPs as well as the depression of polysynaptic VRRs, Renshaw cell discharges and gamma-motoneurone activity induced by meclonazepam (0.1 mg kg-1 i.v.), diazepam (0.3 mg kg-1 i.v.) or zopiclone (1 mg kg-1 i.v.). The same dose of Ro 15-1788 failed to reduce similar effects of phenobarbital (10 mg kg-1 i.v.) on spinal cord activities. (2)In unanaesthetized "encephale isole" rats, 3 mg kg-1 Ro 15-1788 i.v. abolished the decrease induced by 5 mg kg-1 midazolam i.v. of spontaneous multiunit activity (MUA) in the substantia nigra pars compacta, nucleus raphe dorsalis, nucleus locus coeruleus and the CAl area of the hippocampus dorsalis, but not the decrease produced by 10mg kg-1 pentobarbital i.v. Ro 15-1788 (12mg kg-1 i.v.) by itself did not affect MUA in the substantia nigra, but slightly depressed MUA in the other 3 areas. (3) In intact immobilized rats, the increase of power induced by 1 mg kg-1 flunitrazepam i.v. in the 0.5-48 Hz range of the electrocorticogram as well as in the 0.5-8 Hz, 8-32 Hz and 32-48 Hz frequency bands was transiently abolished by 5 mg kg-1 Ro 15-1788 i.v. (4) In unrestrained cats, 5 mg kg-1 Ro 15-1788 i.p. had no effect on the electrical threshold for eliciting a rage reaction evoked by electric hypothalamic stimulation, but abolished the threshold increase caused by 1 mg kg-1 diazepam i.p. These results are in line with biochemical and behavioural findings and support the selective antagonism by Ro 15-1788 of central effects of benzodiazepines through an interaction at benzodiazepine receptors.


