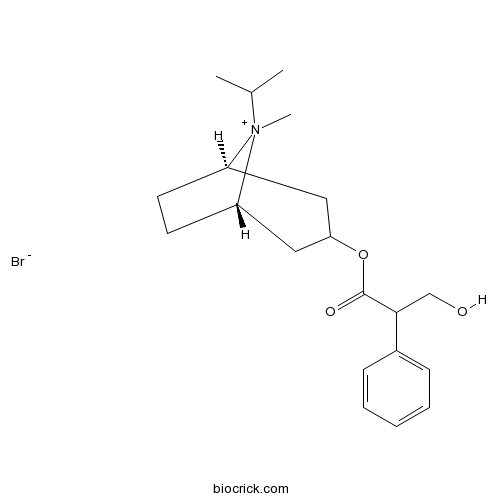
Ipratropium BromideMuscarinic antagonist CAS# 22254-24-6 |
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 22254-24-6 | SDF | Download SDF |
| PubChem ID | 657308 | Appearance | Powder |
| Formula | C20H30BrNO3 | M.Wt | 412.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sch 1000 | ||
| Solubility | H2O : 100 mg/mL (242.51 mM; Need ultrasonic) DMSO : ≥ 35 mg/mL (84.88 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(1S,5R)-8-methyl-8-propan-2-yl-8-azoniabicyclo[3.2.1]octan-3-yl] 3-hydroxy-2-phenylpropanoate;bromide | ||
| SMILES | CC(C)[N+]1(C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3)C.[Br-] | ||
| Standard InChIKey | LHLMOSXCXGLMMN-CLTUNHJMSA-M | ||
| Standard InChI | InChI=1S/C20H30NO3.BrH/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15;/h4-8,14,16-19,22H,9-13H2,1-3H3;1H/q+1;/p-1/t16-,17+,18?,19?,21?; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Muscarinic antagonist, bronchodilator. N-Isopropyl salt of atropine. |

Ipratropium Bromide Dilution Calculator

Ipratropium Bromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.425 mL | 12.125 mL | 24.2501 mL | 48.5001 mL | 60.6252 mL |
| 5 mM | 0.485 mL | 2.425 mL | 4.85 mL | 9.7 mL | 12.125 mL |
| 10 mM | 0.2425 mL | 1.2125 mL | 2.425 mL | 4.85 mL | 6.0625 mL |
| 50 mM | 0.0485 mL | 0.2425 mL | 0.485 mL | 0.97 mL | 1.2125 mL |
| 100 mM | 0.0243 mL | 0.1213 mL | 0.2425 mL | 0.485 mL | 0.6063 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ipratropium bromide is a mAChR M (muscarinic) antagonist, bronchodilator. N-Isopropyl salt of atropine.
- 7-Amino-3-methyl-3-cephem-4-carboxylic acid
Catalog No.:BCC8776
CAS No.:22252-43-3
- Fern-7-en-19-one
Catalog No.:BCN6443
CAS No.:222294-61-3
- Thalrugosaminine
Catalog No.:BCN7745
CAS No.:22226-73-9
- Cucurbitacin I
Catalog No.:BCC2439
CAS No.:2222-07-3
- Dehydroglaucine
Catalog No.:BCN2548
CAS No.:22212-26-6
- Dihydroseselin
Catalog No.:BCN8258
CAS No.:2221-66-1
- Naproxen
Catalog No.:BCC9091
CAS No.:22204-53-1
- Pyrantel Pamoate
Catalog No.:BCC4958
CAS No.:22204-24-6
- Lyn peptide inhibitor
Catalog No.:BCC5895
CAS No.:222018-18-0
- L-Canavanine sulfate
Catalog No.:BCC6746
CAS No.:2219-31-0
- Macrocarpal N
Catalog No.:BCN5811
CAS No.:221899-21-4
- Zotarolimus(ABT-578)
Catalog No.:BCC5481
CAS No.:221877-54-9
- Methyl 6-hydroxyangolensate
Catalog No.:BCN5054
CAS No.:22255-07-8
- alpha-Amyrin palmitate
Catalog No.:BCN5055
CAS No.:22255-10-3
- Guaijaverin
Catalog No.:BCN5056
CAS No.:22255-13-6
- Trimethoxystilbene
Catalog No.:BCN6762
CAS No.:22255-22-7
- Lucidin 3-O-glucoside
Catalog No.:BCN8249
CAS No.:22255-29-4
- Loganic acid
Catalog No.:BCN5057
CAS No.:22255-40-9
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- Tempol
Catalog No.:BCC4862
CAS No.:2226-96-2
- Bromocriptine mesylate
Catalog No.:BCC6642
CAS No.:22260-51-1
- Antidesmone
Catalog No.:BCN5058
CAS No.:222629-77-8
- ACBC
Catalog No.:BCC6584
CAS No.:22264-50-2
- Chysin A
Catalog No.:BCN2020
CAS No.:22269-11-0
Efficacy and safety of ipratropium bromide/salbutamol sulphate administered in a hydrofluoroalkane metered-dose inhaler for the treatment of COPD.[Pubmed:27418820]
Int J Chron Obstruct Pulmon Dis. 2016 Jun 30;11:1469-76.
BACKGROUND: The use of chlorofluorocarbons (CFCs) has contributed to the depletion of the stratospheric ozone layer resulting in serious health concerns. Ipratropium Bromide/salbutamol sulphate CFC-pressurized metered-dose inhalers (IB/SAL-CFC pMDI) have been in widespread use for many years without any apparent ill consequences. This combination has now been reformulated using the hydrofluoroalkane (HFA) propellant. This study sought to establish the clinical noninferiority of a new HFA-containing IB/SAL pMDI to the conventional IB/SAL-CFC pMDI in subjects with mild/moderate COPD. METHODS: This was a randomized, double-blind, parallel-group, multicenter study in two consecutive periods: a 14-day run-in period followed by a 85-day treatment period. Eligible mild-to-moderate stable COPD subjects aged 40-75 years were enrolled into the study and entered the run-in period during which subjects withdrew all the bronchodilators, except for salbutamol as rescue medication. Subjects were randomized to 85 days treatment with either IB/SAL-HFA or IB/SAL-CFC, 20 mug qid. RESULTS: Of the 290 randomized patients, 249 completed the study. The primary efficacy variable was the change in forced expiratory volume in one second from predose to 60 minutes after dosing on day 85. At the end of the treatment period, the adjusted mean change in forced expiratory volume in one second at 60 minutes was 123 mL in the IB/SAL-HFA pMDI group and 115 mL in the IB/SAL-CFC pMDI group. Because the lower limit of the 95% confidence interval for the between-group difference (-62 mL) was well within the noninferiority margin (-100 mL), the HFA formulation was deemed clinically noninferior to the CFC formulation. This finding was supported by secondary efficacy assessments. Both formulations of IB/SAL were well tolerated during the prolonged multiple dosing. CONCLUSION: It is concluded that IB/SAL-HFA pMDI provides effective bronchodilation of similar degree to that achieved with IB/SAL-CFC pMDI. Therefore, IB/SAL-HFA pMDI is a valuable alternative to IB/SAL-CFC pMDI.
Bronchodilator effects of ipratropium bromide and albuterol sulfate among subjects with tetraplegia.[Pubmed:27808011]
J Spinal Cord Med. 2018 Jan;41(1):42-47.
OBJECTIVE: In addition to lung volume restriction, persons with chronic tetraplegia demonstrate obstructive airway physiology evinced by pharmacologically-induced bronchodilation. We previously found independent evidence that anticholinergic agents (Ipratropium Bromide; IB) and beta-2 adrenergic agonists (albuterol sulfate; AS) were associated with significant bronchodilation in subjects with tetraplegia as determined via spirometry or body plethysmography. Direct comparison of these two classes of agents has received little attention. METHODS: Twelve subjects with chronic tetraplegia completed single dose treatment on alternate days with nebulized IB or AS. Patients underwent pre- and 30-minute post-bronchodilator spirometry, body plethysmography, and impulse oscillation system (IOS) in accordance with established protocols. RESULTS: Spirometry and specific airway conductance revealed significant bronchodilator responsiveness following both IB and AS. As determined by increases in specific airway conductance post-bronchodilator, IB tended toward greater bronchodilation than AS (71% vs. 47%). IOS revealed a greater reduction in central airway resistance (R20) following IB compared to AS (22% vs. 9%, P < 0.01). A greater number of subjects exhibited a clinically significant reduction in R20 following IB compared to AS (58% vs. 8%, P < 0.01). CONCLUSION: Among subjects with tetraplegia, both IB and AS elicit significant bronchodilation, although the magnitude of the bronchodilator response is greater following IB. This lends support to theory of overriding cholinergic airway tone in tetraplegia. The IOS findings further suggest that the predominant site of action of IB is upon the larger central airways congruent with findings in able-bodied subjects.
Bilateral acute angle closure developing due to use of ipratropium bromide and salbutamol.[Pubmed:28168569]
Int Ophthalmol. 2018 Feb;38(1):385-388.
Acute angle closure can be seen as a side effect of some medications that can be used systemically. In this article, clinical characteristics of 54-year-old female patient who applied to our clinic with bilateral acute angle closure and has been received nebulized form of salbutamol and Ipratropium Bromide due to asthma for 4 days was evaluated. Right and left eye IOP were measured as 50 and 48 mmHg. IOP was reduced with anti-glaucomatous treatment. and peripheral iridectomy was done, and then the patient was discharged. It is necessary to be careful to prevent contact with the eye of nebulized form of these drugs which may result in angle closure glaucoma when used systemically.
Inhalable Ipratropium Bromide Particle Engineering with Multicriteria Optimization.[Pubmed:27873181]
AAPS PharmSciTech. 2017 Aug;18(6):1925-1935.
Spray-dried Ipratropium Bromide (IPB) microspheres for oral inhalation were engineered using Quality by Design. The interrogation of material properties, process parameters, and critical product quality attributes interplay enabled rational product design. A 2(7-3) screening design exhibited the Maillard reaction between L-leucine (LL) and lactose at studied outlet temperatures (OT) >130 degrees C. A response surface custom design was used in conjunction with multicriteria optimization to determine the operating design space to achieve inhalable microparticles. Statistically significant predictive models were developed for volume median diameter (p = 0.0001, adjusted R (2) = 0.9938), span (p = 0.0278, adjusted R (2) = 0.7912), yield (p = 0.0020, adjusted R (2) = 0.9320), and OT (p = 0.0082, adjusted R (2) = 0.8768). An independent verification batch confirmed the model's predictive capability. The prediction and actual values were in good agreement. Particle size and span were 3.32 +/- 0.09 mum and 1.71 +/- 0.18, which were 4.7 and 5.3% higher than the predicted values. The process yield was 50.3%, compared to the predicted value of 65.3%. The OT was 100 degrees C versus the predicted value of 105 degrees C. The label strength of IPB microparticles was 99.0 to 105.9% w/w suggesting that enrichment occurred during the spray-drying process. The present study can be utilized to initiate the design of the first commercial IPB dry powder inhaler.
Ipratropium bromide potentiates bronchoconstriction induced by vagal nerve stimulation in the guinea-pig.[Pubmed:2958300]
Eur J Pharmacol. 1987 Jul 9;139(2):187-91.
In anaesthetised guinea-pigs, bronchoconstriction induced by vagal nerve stimulation was potentiated by low doses of the antimuscarinic bronchodilator drug, ipratropium (0.01-1.0 microgram/kg); the maximum effect was obtained with 1.0 microgram/kg which doubled the bronchoconstriction. When the dose was increased above 1.0 microgram/kg potentiation no longer occurred; instead the vagally induced bronchoconstriction was antagonised. This was accompanied by reduction in the bronchoconstriction and bradycardia induced by i.v. acetylcholine, due to blockade of post-junctional muscarinic receptors in the airways and heart. With 10 micrograms/kg ipratropium responses elicited both by vagal stimulation and by exogenous acetylcholine were abolished. The results show that ipratropium is an antagonist for pre-junctional muscarinic inhibitory receptors on pulmonary parasympathetic nerves and also confirm its potent antagonist actions on post-junctional muscarinic receptors in the airway smooth muscle. The effect of ipratropium in the lung depends, therefore, on the balance between the pre- and post-junctional effects.


