Noladin etherEndogenous agonist for GPR55 and CB1 CAS# 222723-55-9 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- MMAD
Catalog No.:BCC1774
CAS No.:203849-91-6
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 222723-55-9 | SDF | Download SDF |
| PubChem ID | 6483057 | Appearance | Powder |
| Formula | C23H40O3 | M.Wt | 364.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | HU 310, 2-Arachidonyl glycerol ether | ||
| Solubility | Soluble in ethanol (supplied pre-dissolved in anhydrous ethanol, 5mg/ml) | ||
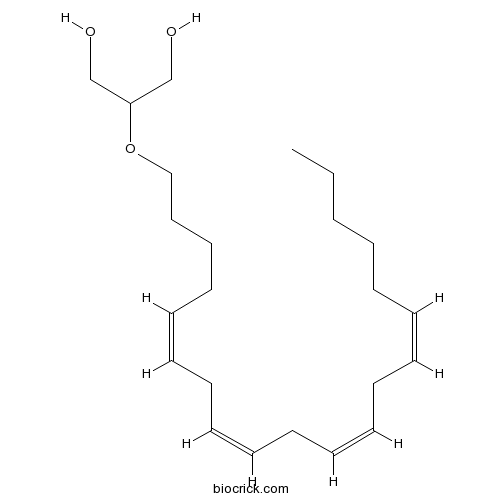
| Chemical Name | 2-[(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoxy]propane-1,3-diol | ||
| SMILES | CCCCCC=CCC=CCC=CCC=CCCCCOC(CO)CO | ||
| Standard InChIKey | CUJUUWXZAQHCNC-DOFZRALJSA-N | ||
| Standard InChI | InChI=1S/C23H40O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-26-23(21-24)22-25/h6-7,9-10,12-13,15-16,23-25H,2-5,8,11,14,17-22H2,1H3/b7-6-,10-9-,13-12-,16-15- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous agonist for the GPR55 and CB1 receptors (EC50 values are 10 and 37 nM respectively) that displays selectivity over CB2 receptors (Ki values are 21.2 nM and > 3mM at CB1 and CB2 respectively). Causes sedation, hypothermia, intestinal immobility and mild antinociception in vivo. Attenuates sensory neurotransmission in rat mesenteric arteries via GPR55. |

Noladin ether Dilution Calculator

Noladin ether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.743 mL | 13.7148 mL | 27.4296 mL | 54.8591 mL | 68.5739 mL |
| 5 mM | 0.5486 mL | 2.743 mL | 5.4859 mL | 10.9718 mL | 13.7148 mL |
| 10 mM | 0.2743 mL | 1.3715 mL | 2.743 mL | 5.4859 mL | 6.8574 mL |
| 50 mM | 0.0549 mL | 0.2743 mL | 0.5486 mL | 1.0972 mL | 1.3715 mL |
| 100 mM | 0.0274 mL | 0.1371 mL | 0.2743 mL | 0.5486 mL | 0.6857 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SZL P1-41
Catalog No.:BCC8004
CAS No.:222716-34-9
- Chysin A
Catalog No.:BCN2020
CAS No.:22269-11-0
- ACBC
Catalog No.:BCC6584
CAS No.:22264-50-2
- Antidesmone
Catalog No.:BCN5058
CAS No.:222629-77-8
- Bromocriptine mesylate
Catalog No.:BCC6642
CAS No.:22260-51-1
- Tempol
Catalog No.:BCC4862
CAS No.:2226-96-2
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- Loganic acid
Catalog No.:BCN5057
CAS No.:22255-40-9
- Lucidin 3-O-glucoside
Catalog No.:BCN8249
CAS No.:22255-29-4
- Trimethoxystilbene
Catalog No.:BCN6762
CAS No.:22255-22-7
- Guaijaverin
Catalog No.:BCN5056
CAS No.:22255-13-6
- alpha-Amyrin palmitate
Catalog No.:BCN5055
CAS No.:22255-10-3
- Finasteride acetate
Catalog No.:BCC4204
CAS No.:222989-99-3
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Evodol
Catalog No.:BCN5059
CAS No.:22318-10-1
- Platycodigenin
Catalog No.:BCN3183
CAS No.:22327-82-8
- Methyl ferulate
Catalog No.:BCN4023
CAS No.:22329-76-6
- 9-Hydroxy-alpha-lapachone
Catalog No.:BCN5060
CAS No.:22333-58-0
- Grandiflorenic acid
Catalog No.:BCN4670
CAS No.:22338-67-6
- Grandifloric acid
Catalog No.:BCN4669
CAS No.:22338-69-8
- Polygalacic acid
Catalog No.:BCN5898
CAS No.:22338-71-2
- FG2216
Catalog No.:BCC6402
CAS No.:223387-75-5
Noladin ether, a putative endocannabinoid, enhances motivation to eat after acute systemic administration in rats.[Pubmed:22309979]
Br J Pharmacol. 2012 Jul;166(6):1815-21.
BACKGROUND AND PURPOSE Endocannabinoid systems are strongly implicated in the physiological control of appetite and eating behaviour, with cannabinoid CB(1) receptor agonists and antagonists, respectively, increasing or decreasing food intake. This study examined the acute actions of the putative endocannabinoid Noladin ether on food intake and eating motivation, assessing how it affects the amount of work expended by animals to obtain food. EXPERIMENTAL APPROACH Non-deprived male rats were injected systemically with Noladin ether to assess its acute effects on ad libitum feeding of a standard laboratory diet. Additionally, the effects of noladin on lever pressing for palatable food were determined using a progressive ratio (PR) operant paradigm. KEY RESULTS Noladin dose dependently increased 2 h food intake, with a significant effect over 1 h after a dose of 0.5 mg.kg(-1). In the PR test, this hyperphagic dose of Noladin ether promoted sustained high rates of responding and significantly increased the total number of lever presses and break-point. These latter effects were prevented by pretreatment with 1.0 mg.kg(-1) of the selective CB(1) antagonist surinabant (SR147778), that alone had no effect on responding. CONCLUSIONS AND IMPLICATIONS This is the first report of hyperphagia induced by acute noladin administration, and the first description of behavioural actions in rats. Consistent with prevailing notions about the role of endocannabinoids in appetite, a hyperphagic dose of noladin markedly increased efforts expended by animals to obtain food. Thus, noladin exerts a specific action on eating motivation; possibly promoting eating by increasing the incentive value of food.
Stimulation of accumbens shell cannabinoid CB(1) receptors by noladin ether, a putative endocannabinoid, modulates food intake and dietary selection in rats.[Pubmed:22728691]
Pharmacol Res. 2012 Sep;66(3):276-82.
Stimulation of cannabinoid CB(1) receptors in nucleus accumbens shell has been shown to stimulate feeding and enhance positive 'liking' reactions to intraoral sucrose. This study examined the behavioural effects of Noladin ether and 2-arachidonoylglycerol following infusion into accumbens shell, on chow intake and food preference in high-carbohydrate and high-fat preferring rats. Noladin ether, potently and dose-dependently stimulated chow intake as compared with 2-arachidonoylglycerol in free-feeding rats. In the diet preference paradigm, in which rats were given free access to both, high-carbohydrate (HC) and high-fat (HF) diets simultaneously, an intra-accumbens administration of Noladin ether as well as 2-arachidonoylglycerol, preferentially enhanced fat consumption over carbohydrate in both HF- and HC-preferring rats. These effects were significantly attenuated by the CB(1) receptor antagonist, AM 251. These results suggesting that, the endocannabinoids through CB(1) receptors, affects appetite for specific dietary components. Both these agents exert a specific action on eating motivation and possibly promoting eating by enhancing the incentive value of food. Altogether these findings reinforce the idea that the endogenous cannabinoid system in the accumbens shell may be important to augment reward-driven feeding via modulation of CB(1) receptor signalling pathways.
Noladin ether acts on trabecular meshwork cannabinoid (CB1) receptors to enhance aqueous humor outflow facility.[Pubmed:16639008]
Invest Ophthalmol Vis Sci. 2006 May;47(5):1999-2005.
PURPOSE: To study the effects of 2-arachidonyl glyceryl ether (Noladin ether), an endocannabinoid ligand selective for cannabinoid (CB)1 receptor, on aqueous humor outflow facility, to investigate the involvement of trabecular meshwork CB1 receptors and the p42/44 MAP kinase signaling pathway and to explore the cellular mechanisms of Noladin ether-induced changes of outflow facility. METHODS: The effects of Noladin ether on aqueous humor outflow facility were measured in a porcine anterior-segment-perfused organ culture model. The expression of CB1 receptors on cultured porcine trabecular meshwork cells and the coupling of these receptors to p42/44 MAP kinase was determined by immunofluorescence microscopy and Western blot analysis. Both Western blot and zymography were used to monitor the effects of Noladin ether on matrix metalloproteinase (MMP)-2. In morphologic studies, AlexaFluor 488-labeled phalloidin staining was used to examine actin filament, and immunohistochemistry with anti-paxillin antibodies was used to detect focal adhesions. RESULTS: Within 1 hour after adding 3, 30, or 300 nM of Noladin ether, the aqueous humor outflow facility increased concentration dependently. The effect of 30 nM of Noladin ether was completely blocked by SR141716A, a selective CB1 antagonist. Positive signals were detected on cultured porcine trabecular meshwork cells with an anti-CB1 antibody in immunofluorescence microscopy and Western blot studies. Treatment of trabecular meshwork cells with 30 nM of Noladin ether activated p42/44 MAP kinase, whereas pretreatment with SR141716A blocked the p42/44 MAP kinase-activating effects of Noladin ether. In addition, the enhancement of outflow facility induced by Noladin ether was blocked by pretreatment of porcine anterior segments with PD98059, an inhibitor of p42/44 MAP kinase pathway. Furthermore, Noladin ether treatment caused rounding of trabecular meshwork cells, and there was a decrease of actin stress fibers, as well as a decrease in focal adhesions. These Noladin ether-induced morphologic changes were also blocked by SR141716A and PD98059. CONCLUSIONS: The results demonstrate for the first time that administration of Noladin ether, an endocannabinoid agonist selective for the CB1 receptor, increases aqueous humor outflow facility. The data also show that Noladin ether-induced enhancement of outflow facility is mediated through the trabecular meshwork CB1 receptor, with an involvement of p42/44 MAP kinase signaling pathway and changes in actin cytoskeletons.
Noladin ether, a putative endocannabinoid, inhibits mu-opioid receptor activation via CB2 cannabinoid receptors.[Pubmed:17698254]
Neurochem Int. 2008 Jan;52(1-2):321-8.
We examined the occurrence of possible changes in mRNA expression and the functional activity of opioid receptors after acute in vivo and in vitro treatment with the putative endogenous cannabinoid Noladin ether. While Noladin ether (NE) demonstrates agonist activity at CB1 cannabinoid receptors, recent data indicate that NE acts as a full agonist at CB2 cannabinoid receptors too. Considering the functional interactions between opioids and cannabinoids, it is of interest to examine whether NE affects the opioid system. To that end, we studied the influence of NE on mu-opioid receptor (MOR) mRNA expression and MOR mediated G-protein signaling. We used real-time PCR and [35S]GTPgammaS binding assays to examine the changes of MOR mRNA levels and the capability of the mu-opioid agonist peptide ([D-Ala2,(NMe)Phe4,Gly5-ol]enkephalin (DAMGO) in activating regulatory G-proteins via MORs in forebrain membrane fractions of wild-type (w.t., CB1+/+) and CB1 receptor deficient transgenic mice (knockout, CB1-/-). We found, that the expression of MOR mRNAs significantly decreased both in CB1+/+ and CB1-/- forebrain after a single injection of NE at 1 mg/kg when compared to control. Consequently, MOR-mediated signaling is attenuated after acute in vivo treatment with NE in both CB1+/+ and CB1-/- mice. Inhibition on MOR mediated activation is observed after in vitro NE administration as well. Radioligand binding competition studies showed that the noticed effect of NE on MOR signaling is not mediated through MORs. Both in vivo and in vitro attenuations of NE can be antagonized by the CB2 selective antagonist SR144528. Taken together, our data suggest that the NE caused pronounced decrease in the activity of MOR is mediated via CB2 cannabinoid receptors.
The orphan receptor GPR55 is a novel cannabinoid receptor.[Pubmed:17876302]
Br J Pharmacol. 2007 Dec;152(7):1092-101.
BACKGROUND: The endocannabinoid system functions through two well characterized receptor systems, the CB1 and CB2 receptors. Work by a number of groups in recent years has provided evidence that the system is more complicated and additional receptor types should exist to explain ligand activity in a number of physiological processes. EXPERIMENTAL APPROACH: Cells transfected with the human cDNA for GPR55 were tested for their ability to bind and to mediate GTPgammaS binding by cannabinoid ligands. Using an antibody and peptide blocking approach, the nature of the G-protein coupling was determined and further demonstrated by measuring activity of downstream signalling pathways. KEY RESULTS: We demonstrate that GPR55 binds to and is activated by the cannabinoid ligand CP55940. In addition endocannabinoids including anandamide and virodhamine activate GTPgammaS binding via GPR55 with nM potencies. Ligands such as cannabidiol and abnormal cannabidiol which exhibit no CB1 or CB2 activity and are believed to function at a novel cannabinoid receptor, also showed activity at GPR55. GPR55 couples to Galpha13 and can mediate activation of rhoA, cdc42 and rac1. CONCLUSIONS: These data suggest that GPR55 is a novel cannabinoid receptor, and its ligand profile with respect to CB1 and CB2 described here will permit delineation of its physiological function(s).
Noladin ether, a putative endocannabinoid, attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a non-CB1/CB2 G(i/o) linked receptor.[Pubmed:15148262]
Br J Pharmacol. 2004 Jun;142(3):509-18.
1 Noladin ether has recently been reported to be an endocannabinoid, with selectivity for the cannabinoid (CB) CB1 receptor. In the present study, we investigated the effects of Noladin ether in the rat isolated mesenteric arterial bed, cultured dorsal root ganglia (DRG) cells and human vanilloid (TRPV1)-receptor-expressing HEK293 cells (TRPV1-HEK293 cells). 2 Electrical field stimulation of the mesenteric bed evoked frequency-dependent vasorelaxation due to the action of calcitonin gene-related peptide (CGRP) released from sensory nerves. Noladin ether (0.1-3 microm) attenuated sensory neurogenic relaxation in a concentration-dependent manner. Noladin ether (1 microm) reduced vasorelaxation at a submaximal frequency (8 Hz), from 57.3+/-6.8 to 23.3+/-3.8% (P<0.05, n=4). 3 The inhibitory effects of Noladin ether were unaffected by the CB1 antagonists SR141716A and LY320135, and the CB2 antagonist SR144528 (1 microm). 4 Noladin ether had no effect on vasorelaxation elicited by exogenous CGRP or capsaicin. These data suggest that Noladin ether is acting at a prejunctional site and no interaction with TRPV1 is involved. 5 In mesenteric beds from pertussis toxin (PTX)-pretreated rats, the inhibitory actions of Noladin ether on sensory neurotransmission were abolished, indicating the involvement of G(i/o) protein-coupled receptors. 6 Noladin ether evoked a concentration-dependent increase in intracellular Ca2+ concentration in TRPV1-HEK293 cells at 10 microm (36.5+/-3.2% of maximal capsaicin-induced response), but it was a less potent agonist than both capsaicin and anandamide and at 1 microm it was essentially inactive. Noladin ether (1 microm) had no effect on capsaicin-evoked Ca2+ responses in DRG cells, and produced no response alone, indicating it neither modulates nor acts directly on TRPV1 receptors. 7 These data demonstrate that Noladin ether attenuates sensory neurotransmission in rat mesenteric arteries via a non-CB1 non-CB2 PTX-sensitive prejunctional site, independently of TRPV1 receptors.
2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor.[Pubmed:11259648]
Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):3662-5.
Two types of endogenous cannabinoid-receptor agonists have been identified thus far. They are the ethanolamides of polyunsaturated fatty acids--arachidonoyl ethanolamide (anandamide) is the best known compound in the amide series--and 2-arachidonoyl glycerol, the only known endocannabinoid in the ester series. We report now an example of a third, ether-type endocannabinoid, 2-arachidonyl glyceryl ether (Noladin ether), isolated from porcine brain. The structure of Noladin ether was determined by mass spectrometry and nuclear magnetic resonance spectroscopy and was confirmed by comparison with a synthetic sample. It binds to the CB(1) cannabinoid receptor (K(i) = 21.2 +/- 0.5 nM) and causes sedation, hypothermia, intestinal immobility, and mild antinociception in mice. It binds weakly to the CB(2) receptor (K(i) > 3 microM).
Synthesis and biological activities of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, and its metabolically stable ether-linked analogues.[Pubmed:10923815]
Chem Pharm Bull (Tokyo). 2000 Jul;48(7):903-7.
We synthesized 2-arachidonoylglycerol (1), an endogenous cannabinoid receptor ligand, and its metabolically stable ether-linked analogues. Compound 1 was synthesized from 1,3-benzylideneglycerol (6) and arachidonic acid in the presence of N,N'-dicyclohexylcarbodiimide and 4-dimethylaminopyridine followed by treatment with boric acid and trimethyl borate. An ether-linked analogue of 2-arachidonoylglycerol (2) was synthesized from 6 and 5,8,11,14-eicosatetraenyl iodide (9). The ether-linked analogues of 2-palmitoylglycerol (4) and 2-oleoyglycerol (5) were synthesized from 6 and hexadecyl iodide (12) and 9-octadecenyl iodide (14), respectively. We confirmed that 1 stimulates NG108-15 cells to induce rapid transient elevation of the intracellular free Ca2+ concentrations through a CB1 receptor-dependent mechanism. Noticeably, 2 exhibited appreciable agonistic activity, although its activity was significantly lower than that of 1. Compound 2 would be a useful tool in exploring the physiological significance of 1, because this compound is resistant to hydrolyzing enzymes in contrast to 1. On the other hand, the ether-linked analogues of either 4 or 5 failed to act as a CB1 receptor agonist. Compounds 4 and 5 would also be valuable as control molecules in experiments where 2 is employed.
Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds.[Pubmed:9915812]
J Biol Chem. 1999 Jan 29;274(5):2794-801.
An endogenous cannabimimetic molecule, 2-arachidonoylglycerol, induces a rapid, transient increase in intracellular free Ca2+ concentrations in NG108-15 cells through a cannabinoid CB1 receptor-dependent mechanism. We examined the activities of 24 relevant compounds (2-arachidonoylglycerol, its structural analogues, and several synthetic cannabinoids). We found that 2-arachidonoylglycerol is the most potent compound examined so far: its activity was detectable from as low as 0.3 nM, and the maximal response induced by 2-arachidonoylglycerol exceeded the responses induced by others. Activities of HU-210 and CP55940, potent cannabinoid receptor agonists, were also detectable from as low as 0.3 nM, whereas the maximal responses induced by these compounds were low compared with 2-arachidonoylglycerol. Anandamide was also found to act as a partial agonist in this assay system. We confirmed that free arachidonic acid failed to elicit a response. Furthermore, we found that a metabolically stable ether-linked analogue of 2-arachidonoylglycerol possesses appreciable agonistic activity, although its activity was apparently lower than that of 2-arachidonoylglycerol. We also confirmed that pretreating cells with various cannabinoid receptor agonists nullified the response induced by 2-arachidonoylglycerol, whereas pretreating cells with other neurotransmitters or neuromodulators did not affect the response. These results strongly suggested that the cannabinoid CB1 receptor is originally a 2-arachidonoylglycerol receptor, and 2-arachidonoylglycerol is the intrinsic physiological ligand for the cannabinoid CB1 receptor.


