pep2-EVKIPeptide inhibitor of GluR2 subunit binding to PICK1 CAS# 1315378-67-6 |
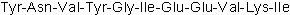
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- CGS 21680 HCl
Catalog No.:BCC4316
CAS No.:124431-80-7
- Valsartan
Catalog No.:BCC5017
CAS No.:137862-53-4
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- Ticlopidine HCl
Catalog No.:BCC4973
CAS No.:53885-35-1
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure
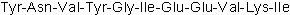
3D structure
| Cas No. | 1315378-67-6 | SDF | Download SDF |
| PubChem ID | 90479809 | Appearance | Powder |
| Formula | C62H95N13O19 | M.Wt | 1326.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in PBS (pH6.8) | ||
| Sequence | YNVYGIEEVKI | ||
| Chemical Name | (2S,3S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-4-oxobutanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]acetyl]amino]-3-methylpentanoyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]-3-methylbutanoyl]amino]hexanoyl]amino]-3-methylpentanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NC(C(C)CC)C(=O)O)NC(=O)CNC(=O)C(CC1=CC=C(C=C1)O)NC(=O)C(C(C)C)NC(=O)C(CC(=O)N)NC(=O)C(CC2=CC=C(C=C2)O)N | ||
| Standard InChIKey | KGQYILLVACJAQP-XMCCVONBSA-N | ||
| Standard InChI | InChI=1S/C62H95N13O19/c1-9-33(7)51(61(92)69-41(22-24-47(80)81)55(86)67-42(23-25-48(82)83)57(88)73-49(31(3)4)59(90)68-40(13-11-12-26-63)56(87)75-52(62(93)94)34(8)10-2)72-46(79)30-66-54(85)43(28-36-16-20-38(77)21-17-36)71-60(91)50(32(5)6)74-58(89)44(29-45(65)78)70-53(84)39(64)27-35-14-18-37(76)19-15-35/h14-21,31-34,39-44,49-52,76-77H,9-13,22-30,63-64H2,1-8H3,(H2,65,78)(H,66,85)(H,67,86)(H,68,90)(H,69,92)(H,70,84)(H,71,91)(H,72,79)(H,73,88)(H,74,89)(H,75,87)(H,80,81)(H,82,83)(H,93,94)/t33-,34-,39-,40-,41-,42-,43-,44-,49-,50-,51-,52-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor peptide that selectively disrupts binding of the AMPA receptor subunit GluA2 (at the C-terminal PDZ site) to protein interacting with C kinase (PICK1). Does not affect binding of GluA2 to GRIP or ABP and does not increase AMPA current amplitude or affect long term depression (LTD). |

pep2-EVKI Dilution Calculator

pep2-EVKI Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NG25
Catalog No.:BCC1799
CAS No.:1315355-93-1
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- Amyloid Beta-Peptide (1-40) (human)
Catalog No.:BCC1045
CAS No.:131438-79-4
- Cercosporamide
Catalog No.:BCC2438
CAS No.:131436-22-1
- UNC669
Catalog No.:BCC3997
CAS No.:1314241-44-5
- TC-N 22A
Catalog No.:BCC6150
CAS No.:1314140-00-5
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Boc-Ile-OH.1/2H2O
Catalog No.:BCC3406
CAS No.:13139-16-7
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- Triumbelletin
Catalog No.:BCN6779
CAS No.:131559-54-1
- Camelliaside B
Catalog No.:BCN3872
CAS No.:131573-90-5
Emergence of Endocytosis-Dependent mGlu1 LTD at Nucleus Accumbens Synapses After Withdrawal From Cocaine Self-Administration.[Pubmed:30459590]
Front Synaptic Neurosci. 2018 Oct 23;10:36.
Extended-access cocaine self-administration induces a progressive intensification of cue-induced drug craving during withdrawal termed "incubation of cocaine craving". Rats evaluated after >1 month of withdrawal (when incubation of craving is robust) display alterations in excitatory synapses onto medium spiny neurons (MSNs) of the nucleus accumbens (NAc), including elevated levels of Ca(2+)-permeable AMPA receptors (CP-AMPAR) and a transition from group I metabotropic glutamate receptor (mGluR) mGlu5- to mGlu1-mediated synaptic depression. It is important to further characterize the emergent form of mGlu1-mediated synaptic depression because it has been demonstrated that mGlu1 stimulation, by normalizing CP-AMPAR transmission, reduces cue-induced cocaine craving. In the present study, we conducted whole-cell patch-clamp recordings in NAc core MSNs, comparing rats that underwent >35 days of withdrawal from cocaine self-administration to control rats that had self-administered saline. Bath application of the nonselective group I mGluR agonist dihydroxyphenylglycine (DHPG) produced a transient mGlu5-mediated synaptic depression in saline controls, whereas a persistent mGlu1-mediated synaptic depression emerged in cocaine rats. This form of long-term depression (LTD) was abolished by the inclusion of dynamin inhibitory peptide (DIP) in the recording electrode, indicating that it is mediated by removal of CP-AMPARs through a dynamin-dependent endocytosis mechanism. We further showed that CP-AMPAR endocytosis is normally coupled to the PICK1-mediated insertion of Ca(2+)-impermeable AMPARs (CI-AMPAR). Interestingly, this coupling is not obligatory because disruption of PICK1-mediated CI-AMPAR insertion with pep2-EVKI spared mGlu1-mediated CP-AMPAR endocytosis. Collectively, these results reveal similarities but also differences from mGlu1-LTD observed in other brain regions, and further our understanding of a form of plasticity that may be targeted to reduce cue-induced craving for cocaine and methamphetamine.
PDZ protein mediated activity-dependent LTP/LTD developmental switch at rat retinocollicular synapses.[Pubmed:20457829]
Am J Physiol Cell Physiol. 2010 Jun;298(6):C1572-82.
The insertion of amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors into the plasma membrane and removal via internalization are essential for regulating synaptic strength, which underlies the basic mechanism of learning and memory. The retinocollicular pathway undergoes synaptic refinement during development and shows a wide variety of long-term synaptic changes; however, still little is known about its underlying molecular regulation. Here we report a rapid developmental long-term potentiation (LTP)/long-term depression (LTD) switch and its intracellular mechanism at the rat retinocollicular pathway from postnatal day 5 (P5) to P14. Before P9, neurons always exhibited LTP, whereas LTD was observed only after P10. Blockade of GluR2/3-glutamate receptor-interacting protein (GRIP)/AMPA-receptor-binding protein (ABP)/protein interacting with C kinase 1 (PICK1) interactions with pep2-SVKI could sustain the LTP after P10. This suggests that the LTP/LTD switch relied on PDZ protein activities. Selective interruption of GluR2/3-PICK1 binding by pep2-EVKI blocked the long-lasting effects of both LTP and LTD, suggesting a role for PICK1 in the maintenance of long-term synaptic plasticity. Interestingly, synaptic expression of GRIP increased more than twofold from P7 to P11, whereas ABP and PICK1 expression levels remained stable. Blockade of spontaneous retinal input suppressed this increase and abolished the LTP/LTD switch. These results suggest that the increased GRIP synaptic expression may be a key regulatory factor in mediating the activity-dependent developmental LTP/LTD switch, whereas PICK1 may be required for both LTP and LTD to maintain their long-term effects.
Functional roles of protein interactions with AMPA and kainate receptors.[Pubmed:12941441]
Neurosci Res. 2003 Sep;47(1):3-15.
The glutamate receptor subtypes AMPA and kainate are involved in synaptic transmission and synaptic plasticity in the CNS. Recently there has been considerable interest in understanding the molecular regulation of these receptors by proteins that directly bind to AMPA and kainate receptor subunits. Amongst the first interaction partners to be discovered were NSF, ABP, GRIP and PICK1, which bind the AMPA receptor subunit GLUA2. We have studied the functional roles of the interactions of these proteins in regulating AMPA receptor-mediated synaptic transmission and synaptic plasticity in the hippocampus. We have also started to investigate the functions of PICK1 and GRIP on kainate receptor-mediated synaptic transmission in this region. In this article we reflect upon this work, which has led to some new ideas about how AMPA and kainate receptors are regulated at synapses.
NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex.[Pubmed:11931741]
Neuron. 2002 Mar 28;34(1):53-67.
AMPA receptor (AMPAR) trafficking is crucial for synaptic plasticity that may be important for learning and memory. NSF and PICK1 bind the AMPAR GluR2 subunit and are involved in trafficking of AMPARs. Here, we show that GluR2, PICK1, NSF, and alpha-/beta-SNAPs form a complex in the presence of ATPgammaS. Similar to SNARE complex disassembly, NSF ATPase activity disrupts PICK1-GluR2 interactions in this complex. Alpha- and beta-SNAP have differential effects on this reaction. SNAP overexpression in hippocampal neurons leads to corresponding changes in AMPAR trafficking by acting on GluR2-PICK1 complexes. This demonstrates that the previously reported synaptic stabilization of AMPARs by NSF involves disruption of GluR2-PICK1 interactions. Furthermore, we are reporting a non-SNARE substrate for NSF disassembly activity.
PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses.[Pubmed:11163273]
Neuron. 2000 Dec;28(3):873-86.
We investigated the role of PDZ proteins (GRIP, ABP, and PICK1) interacting with the C-terminal GluR2 by infusing a ct-GluR2 peptide ("pep2-SVKI") into CA1 pyramidal neurons in hippocampal slices using whole-cell recordings. Pep2-SVKI, but not a control or PICK1 selective peptide, caused AMPAR-mediated EPSC amplitude to increase in approximately one-third of control neurons and in most neurons following the prior induction of LTD. Pep2-SVKI also blocked LTD; however, this occurred in all neurons. A PKC inhibitor prevented these effects of pep2-SVKI on synaptic transmission and LTD. We propose a model in which the maintenance of LTD involves the binding of AMPARs to PDZ proteins to prevent their reinsertion. We also present evidence that PKC regulates AMPAR reinsertion during dedepression.
AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses.[Pubmed:10526335]
Nat Neurosci. 1999 Nov;2(11):972-7.
Silent synapses form between some primary sensory afferents and dorsal horn neurons in the spinal cord. Molecular mechanisms for activation or conversion of silent synapses to conducting synapses are unknown. Serotonin can trigger activation of silent synapses in dorsal horn neurons by recruiting AMPA receptors. AMPA-receptor subunits GluR2 and GluR3 interact via their cytoplasmic C termini with PDZ-domain-containing proteins such as GRIP (glutamate receptor interacting protein), but the functional significance of these interactions is unclear. Here we demonstrate that protein interactions involving the GluR2/3 C terminus are important for serotonin-induced activation of silent synapses in the spinal cord. Furthermore, PKC is a necessary and sufficient trigger for this activation. These results implicate AMPA receptor-PDZ interactions in mechanisms underlying sensory synaptic potentiation and provide insights into the pathogenesis of chronic pain.


