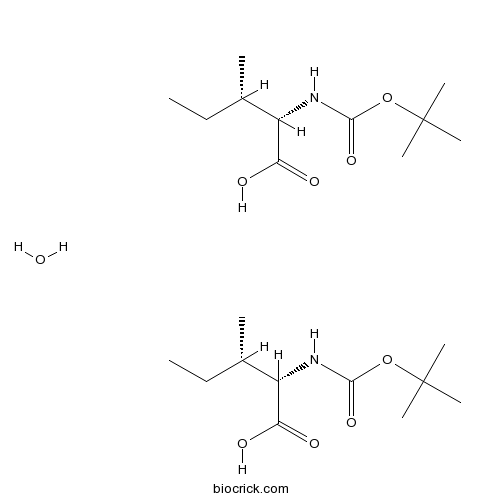
Boc-Ile-OH.1/2H2OCAS# 13139-16-7 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 13139-16-7 | SDF | Download SDF |
| PubChem ID | 45072434 | Appearance | Powder |
| Formula | C22H44N2O9 | M.Wt | 480.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S)-3-methyl-2-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid;hydrate | ||
| SMILES | CCC(C)C(C(=O)O)NC(=O)OC(C)(C)C.CCC(C)C(C(=O)O)NC(=O)OC(C)(C)C.O | ||
| Standard InChIKey | NYGJSARLXBZKTA-AQHOMMPKSA-N | ||
| Standard InChI | InChI=1S/2C11H21NO4.H2O/c2*1-6-7(2)8(9(13)14)12-10(15)16-11(3,4)5;/h2*7-8H,6H2,1-5H3,(H,12,15)(H,13,14);1H2/t2*7-,8-;/m00./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Ile-OH.1/2H2O Dilution Calculator

Boc-Ile-OH.1/2H2O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0807 mL | 10.4037 mL | 20.8073 mL | 41.6146 mL | 52.0183 mL |
| 5 mM | 0.4161 mL | 2.0807 mL | 4.1615 mL | 8.3229 mL | 10.4037 mL |
| 10 mM | 0.2081 mL | 1.0404 mL | 2.0807 mL | 4.1615 mL | 5.2018 mL |
| 50 mM | 0.0416 mL | 0.2081 mL | 0.4161 mL | 0.8323 mL | 1.0404 mL |
| 100 mM | 0.0208 mL | 0.104 mL | 0.2081 mL | 0.4161 mL | 0.5202 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Ile-OH•1/2H2O
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- Boc-Trp-OH
Catalog No.:BCC3455
CAS No.:13139-14-5
- Teijin compound 1
Catalog No.:BCC6057
CAS No.:1313730-14-1
- Periplocin
Catalog No.:BCN2655
CAS No.:13137-64-9
- erythro-Guaiacylglycerol beta-threo-syringylglycerol ether
Catalog No.:BCN7333
CAS No.:1313434-74-0
- NVP-CGM097
Catalog No.:BCC5395
CAS No.:1313363-54-0
- Nystose
Catalog No.:BCN5397
CAS No.:13133-07-8
- threo Ifenprodil hemitartrate
Catalog No.:BCC7508
CAS No.:1312991-83-5
- Ro 8-4304 hydrochloride
Catalog No.:BCC7655
CAS No.:1312991-77-7
- Ro 25-6981 Maleate
Catalog No.:BCC4159
CAS No.:1312991-76-6
- Scutebarbatine Z
Catalog No.:BCN6991
CAS No.:1312716-28-1
- Scutebarbatine Y
Catalog No.:BCN6994
CAS No.:1312716-27-0
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
- TC-N 22A
Catalog No.:BCC6150
CAS No.:1314140-00-5
- UNC669
Catalog No.:BCC3997
CAS No.:1314241-44-5
- Cercosporamide
Catalog No.:BCC2438
CAS No.:131436-22-1
- Amyloid Beta-Peptide (1-40) (human)
Catalog No.:BCC1045
CAS No.:131438-79-4
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- NG25
Catalog No.:BCC1799
CAS No.:1315355-93-1
- pep2-EVKI
Catalog No.:BCC5786
CAS No.:1315378-67-6
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
Metabolic plasticity for subcutaneous fat accumulation in a long-distance migratory bird traced by (2)H2O.[Pubmed:28082618]
J Exp Biol. 2017 Mar 15;220(Pt 6):1072-1078.
The migrant black-tailed godwit (Limosa limosa) traditionally used natural wetlands in the Iberian Peninsula to prepare for migratory flights by feeding mainly in estuaries. In recent decades, this species has become increasingly dependent on rice fields, thereby relying on a plant-based diet for fuelling. Dietary fatty acids (FA) seem to be determinant to the composition of accumulated subcutaneous fat in migratory birds. It is still unclear whether metabolic plasticity allows for modification and/or synthesis of FA, contributing to a lipid profile that enables a successful migratory performance. Deuterated water was administered to captive black-tailed godwits submitted to two diets (fly larvae versus rice) and the incorporation of deuterium ((2)H) into subcutaneous triglycerides was analyzed by NMR. A recently developed localized biopsy method for sampling subcutaneous fat was employed with later successful release of all birds into the wild. The average chemical structure reflected mostly a mixture of saturated and monounsaturated 16- and 18-carbon FA, a profile frequently found in migrant birds. Significantly higher levels of polyunsaturated FA, as well as detectable levels of n-3 FA, were observed in fly-larvae-fed birds. Excess (2)H-enrichments in FA revealed significantly higher rates of fractional de novo lipogenesis and FA desaturation capacity in rice-fed birds. This novel and non-lethal tracer method revealed the capacity of this species to alter its lipid metabolism to compensate for a poorer dietary lipid contribution. Because of its versatility, adapting this method to other scenarios and/or other migratory species is considered feasible and cost-effective.
Proton conduction mechanisms in the phosphoric acid-water system (H4P2O7-H3PO4.2H2O): a (1)H, (31)P and (17)O PFG-NMR and conductivity study.[Pubmed:27918028]
Phys Chem Chem Phys. 2016 Dec 21;19(1):587-600.
Ionic charge carrier formation and mobility, including the underlying conduction mechanisms, are investigated for phosphoric acid at water contents relevant for the acid's application as electrolyte in fuel cells. The high conductivity contribution from structural diffusion involving intermolecular proton transfer ( approximately 97%) in neat phosphoric acid (H3PO4) passes through a maximum at this composition. Hydrogen bond network frustration (imbalance of the number of proton donors and acceptors), which is closely related to the appearance of structural diffusion, decreases with both elimination and addition of water. Structural diffusion is virtually disappearing for H3PO4.2H2O, yet, the overall conductivity increases with increasing water content and reaches a maximum at a composition of H3PO4.5H2O. The conductivity increase is a consequence of the progressive de-coupling of the diffusion of aqueous species from that of phosphate species and the strongly enhanced acidity of phosphoric acid at low water contents. High concentrations of protonated aqueous species with high diffusivity then lead to high conductivity contributions from vehicular transport. The increased water transport associated with the change in transport mechanism is suggested to have severe implications for fuel cell applications. At low water contents the conductivity contribution of structural diffusion is also reduced, but it is accompanied by conductivity contributions from a high concentration of multiply charged condensation products (e.g. H2P2O7(2-), H3P3O10(2-) and H2P3O10(3-)). The results underline the singularity of structure diffusion in neat phosphoric acid (H3PO4) and its sensitivity against any perturbation.
Redetermination of metarossite, CaV(5+)2O6.2H2O.[Pubmed:27920917]
Acta Crystallogr E Crystallogr Commun. 2016 Aug 9;72(Pt 9):1280-1284.
The crystal structure of metarossite, ideally CaV2O6.2H2O [chemical name: calcium divanadium(V) hexa-oxide dihydrate], was first determined using precession photographs, with fixed isotropic displacement parameters and without locating the positions of the H atoms, leading to a reliability factor R = 0.11 [Kelsey & Barnes (1960 black triangle right). Can. Mineral.6, 448-466]. This communication reports a structure redetermination of this mineral on the basis of single-crystal X-ray diffraction data of a natural sample from the Blue Cap mine, San Juan County, Utah, USA (R1 = 0.036). Our study not only confirms the structural topology reported in the previous study, but also makes possible the refinement of all non-H atoms with anisotropic displacement parameters and all H atoms located. The metarossite structure is characterized by chains of edge-sharing [CaO8] polyhedra parallel to [100] that are themselves connected by chains of alternating [VO5] trigonal bipyramids parallel to [010]. The two H2O mol-ecules are bonded to Ca. Analysis of the displacement parameters show that the [VO5] chains librate around [010]. In addition, we measured the Raman spectrum of metarossite and compared it with IR and Raman data previously reported. Moreover, heating of metarossite led to a loss of water, which results in a transformation to the brannerite-type structure, CaV2O6, implying a possible dehydration pathway for the compounds M(2+)V2O6.xH2O, with M = Cu, Cd, Mg or Mn, and x = 2 or 4.
Proton conducting system (ImH2)2SeO4.2H2O investigated with vibrational spectroscopy.[Pubmed:28315619]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 Jun 5;180:224-233.
Imidazolium selenate dihydrate (ImH2)2SeO4.2H2O crystals have been investigated using Raman and IR spectroscopy. Experimental data were supported by the quantum-chemical calculations (DFT), Hirshfield surfaces and fingerprint plots analysis, and Bader theory calculations. The imidazolium selenate dihydrate crystal exhibits high proton conductivity of the order of ~10(-1)S/m at T=333K. The spectra of this compound are dominated by bands related to the lattice modes, the internal vibrations of the protonated imidazole cation, selenate anion, water molecules, and hydrogen bonds network. For the imidazolium selenate dihydrate crystal, the formal classification of the fundamental modes has been carried out.


