Urotensin II (human)Endogenous vasoactive agonist for the UT receptor CAS# 251293-28-4 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
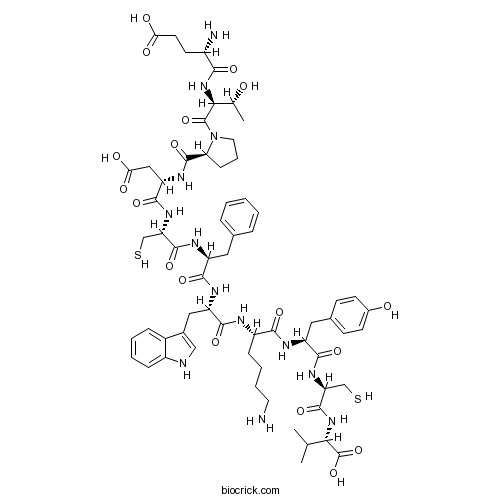
| Cas No. | 251293-28-4 | SDF | Download SDF |
| PubChem ID | 102179738 | Appearance | Powder |
| Formula | C64H85N13O18S2 | M.Wt | 1388.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O Peptide Solubility and Storage Guidelines: 1. Calculate the length of the peptide. 2. Calculate the overall charge of the entire peptide according to the following table: 3. Recommended solution: | ||
| Sequence | ETPDCFWKYCV (Modifications: Disulfide bridge between 5 - 10) | ||
| Chemical Name | (4S)-4-amino-5-[[(2S,3R)-1-[(2S)-2-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2R)-1-[[(1S)-1-carboxy-2-methylpropyl]amino]-1-oxo-3-sulfanylpropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxo-3-sulfanylpropan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-3-hydroxy-1-oxobutan-2-yl]amino]-5-oxopentanoic acid | ||
| SMILES | CC(C)C(C(=O)O)NC(=O)C(CS)NC(=O)C(CC1=CC=C(C=C1)O)NC(=O)C(CCCCN)NC(=O)C(CC2=CNC3=CC=CC=C32)NC(=O)C(CC4=CC=CC=C4)NC(=O)C(CS)NC(=O)C(CC(=O)O)NC(=O)C5CCCN5C(=O)C(C(C)O)NC(=O)C(CCC(=O)O)N | ||
| Standard InChIKey | OFADXNWQLHHILF-USJMABIRSA-N | ||
| Standard InChI | InChI=1S/C64H87N13O18S2/c1-33(2)52(64(94)95)75-61(91)48(32-97)74-57(87)44(27-36-18-20-38(79)21-19-36)69-55(85)42(16-9-10-24-65)68-58(88)45(28-37-30-67-41-15-8-7-14-39(37)41)71-56(86)43(26-35-12-5-4-6-13-35)70-60(90)47(31-96)73-59(89)46(29-51(82)83)72-62(92)49-17-11-25-77(49)63(93)53(34(3)78)76-54(84)40(66)22-23-50(80)81/h4-8,12-15,18-21,30,33-34,40,42-49,52-53,67,78-79,96-97H,9-11,16-17,22-29,31-32,65-66H2,1-3H3,(H,68,88)(H,69,85)(H,70,90)(H,71,86)(H,72,92)(H,73,89)(H,74,87)(H,75,91)(H,76,84)(H,80,81)(H,82,83)(H,94,95)/t34-,40+,42+,43+,44+,45+,46+,47+,48+,49+,52+,53+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent endogenous peptide agonist for the urotensin-II receptor (EC50 = 0.1 nM). Displays arterio-selective vasoconstriction and vasodilatation in mammals in vitro and in vivo, effects which vary between species. Also has been shown to mediate bronchoconstriction. |

Urotensin II (human) Dilution Calculator

Urotensin II (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Crebanine
Catalog No.:BCN5117
CAS No.:25127-29-1
- FRATide
Catalog No.:BCC5821
CAS No.:251087-38-4
- Antazoline HCl
Catalog No.:BCC4627
CAS No.:2508-72-7
- Salvisyrianone
Catalog No.:BCN4821
CAS No.:250691-57-7
- NNC 63-0532
Catalog No.:BCC7177
CAS No.:250685-44-0
- Pedunsaponin A
Catalog No.:BCN8192
CAS No.:250613-27-5
- Cyclo(RGDyK)
Catalog No.:BCC6512
CAS No.:250612-42-1
- (±)-Acetylcarnitine chloride
Catalog No.:BCC6617
CAS No.:2504-11-2
- Excavatin M
Catalog No.:BCN5116
CAS No.:250293-31-3
- PNU 177864 hydrochloride
Catalog No.:BCC7664
CAS No.:250266-51-4
- Boc-His(Dnp)-OH
Catalog No.:BCC3401
CAS No.:25024-53-7
- Otophylloside F
Catalog No.:BCN6441
CAS No.:250217-73-3
- SU 16f
Catalog No.:BCC7639
CAS No.:251356-45-3
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Tesaglitazar
Catalog No.:BCC7828
CAS No.:251565-85-2
- 5-Chloro-2-nitrobenzoic acid
Catalog No.:BCC8743
CAS No.:2516-95-2
- Acevaltrate
Catalog No.:BCN7127
CAS No.:25161-41-5
- Loline
Catalog No.:BCN2003
CAS No.:25161-91-5
- Isohomoarbutin
Catalog No.:BCN7612
CAS No.:25162-30-5
- Dynamin inhibitory peptide
Catalog No.:BCC1034
CAS No.:251634-21-6
- AM 1172
Catalog No.:BCC7675
CAS No.:251908-92-6
- SLV 320
Catalog No.:BCC7656
CAS No.:251945-92-3
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- AZD7545
Catalog No.:BCC4294
CAS No.:252017-04-2
The effects of urotensin II on migration and invasion are mediated by NADPH oxidase-derived reactive oxygen species through the c-Jun N-terminal kinase pathway in human hepatoma cells.[Pubmed:27988353]
Peptides. 2017 Feb;88:106-114.
AIMS: Urotensin II (UII) is a vasoactive neuropeptide involved in migration and invasion in various cell types. However, the effects of UII on human hepatoma cells still remain unclear. The aim of this study was to investigate the role and mechanism of UII on migration and invasion in human hepatoma cells. METHODS: Migration was measured by wound healing assays and a Transwell((R)) methodology, and invasion was analyzed using Matrigel((R)) invasion chambers. Reactive oxygen species (ROS) levels were detected using a 2', 7'-dichlorofluorescein diacetate probe, and flow cytometry, and protein expression levels were evaluated by western blotting. Cell proliferation and actin polymerization were examined using cell proliferation reagent WST-1 and F-actin immunohistochemistry staining. RESULTS: Exposure to UII promoted migration and invasion in hepatoma cells compared with that in cells without UII. UII also increased matrix metalloproteinase-2 (MMP2) expression in a time-independent manner. Furthermore, UII markedly enhanced ROS generation and NADPH oxidase subunit expression, and consequently facilitated the phosphorylation of c-Jun N-terminal kinase (JNK). The UT antagonist urantide or the antioxidant/NADPH oxidase inhibitor apocynin decreased UII-induced ROS production. JNK phosphorylation, migration, invasion, and MMP9/2 expression were also reversed by pretreatment with apocynin. Urantide and JNK inhibitor SP600125 abrogated migration, invasion, or MMP9/2 expression in response to UII. UII induced actin polymerization and fascin protein expression, and could be reversed by apocynin and SP600125. CONCLUSIONS: Exogenous UII induced migration and invasion in hepatoma cells that mainly involved NADPH oxidase-derived ROS through JNK activation. UT played an additional role in regulating hepatoma cells migration and invasion. Thus, our data suggested an important effect of UII in hepatocellular carcinoma metastasis.
Use of Diverse Chemometric and Validation Methods to Accurately Predict Human Urotensin-II Receptor Antagonist Activity.[Pubmed:26694105]
Curr Comput Aided Drug Des. 2015;11(4):361-73.
Despite being identified as the most potent receptor related to vasoconstriction, human urotensin-II receptor (hUT) has not been fully explored as a target for the treatment of cardiovascular diseases. In view of this and with an aim to identify precise structural requirements for binding of hUT antagonists, we endeavoured to develop, for the first time, multivariate QSAR models using chemometric methods like partial least squares (PLS) and feed-forward neural network (FFNN). A set of 48 pyrrolidine derivatives having hUT binding affinity was used for multivariate model development. The accuracy and predictability of the developed models was evaluated using crossvalidation. The PLS model showed good correlation between selected descriptors and Ki values (r(2) =0.745 and r(2) (CV) =0.773). However, the predictive performance of FFNN was better than the PLS technique with r(2) =0.810. The study clearly suggests the role of lipophilic and steric descriptors in the ligand-hUT interactions. The QSAR models generated can be successfully extended to predict the binding affinities and for the effective design of novel hUT antagonists.
Conformation and Dynamics of Human Urotensin II and Urotensin Related Peptide in Aqueous Solution.[Pubmed:28055189]
J Chem Inf Model. 2017 Feb 27;57(2):298-310.
Conformation and dynamics of the vasoconstrictive peptides human urotensin II (UII) and urotensin related peptide (URP) have been investigated by both unrestrained and enhanced-sampling molecular-dynamics (MD) simulations and NMR spectroscopy. These peptides are natural ligands of the G-protein coupled urotensin II receptor (UTR) and have been linked to mammalian pathophysiology. UII and URP cannot be characterized by a single structure but exist as an equilibrium of two main classes of ring conformations, open and folded, with rapidly interchanging subtypes. The open states are characterized by turns of various types centered at K(8)Y(9) or F(6)W(7) predominantly with no or only sparsely populated transannular hydrogen bonds. The folded conformations show multiple turns stabilized by highly populated transannular hydrogen bonds comprising centers F(6)W(7)K(8) or W(7)K(8)Y(9). Some of these conformations have not been characterized previously. The equilibrium populations that are experimentally difficult to access were estimated by replica-exchange MD simulations and validated by comparison of experimental NMR data with chemical shifts calculated with density-functional theory. UII exhibits approximately 72% open:28% folded conformations in aqueous solution. URP shows very similar ring conformations as UII but differs in an open:folded equilibrium shifted further toward open conformations (86:14) possibly arising from the absence of folded N-terminal tail-ring interaction. The results suggest that the different biological effects of UII and URP are not caused by differences in ring conformations but rather by different interactions with UTR.
Autocrine Human Urotensin II Enhances Macrophage-Derived Foam Cell Formation in Transgenic Rabbits.[Pubmed:26640798]
Biomed Res Int. 2015;2015:843959.
Circulating urotensin II (UII) is involved in the development of atherosclerosis. However, the role of autocrine UII in the development of atherosclerosis remains unclear. Here, we tested the hypothesis that autocrine UII would promote atherosclerosis. Transgenic rabbits were created as a model to study macrophage-specific expressing human UII (hUII) and used to investigate the role of autocrine UII in the development of atherosclerosis. Transgenic rabbits and their nontransgenic littermates were fed a high cholesterol diet to induce atherosclerosis. Comparing the transgenic rabbits with their nontransgenic littermates, it was observed that hUII expression increased the macrophage-positive area in the atherosclerotic lesions by 45% and the positive area ratio by 56% in the transgenic rabbits. Autocrine hUII significantly decreased the smooth muscle cell-positive area ratio in transgenic rabbits (by 54%), without affecting the plasma levels of total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and glucose and adipose tissue contents. These results elucidated for the first time that autocrine UII plays an important role in the development of atherosclerosis by increasing the accumulation of macrophage-derived foam cell.
Is urotensin-II the new endothelin?[Pubmed:12381671]
Br J Pharmacol. 2002 Nov;137(5):579-88.
Urotensin-II (U-II), a peptide isolated from the urophysis of teleost fish 35 years ago, is the endogenous ligand of the mammalian orphan receptor GPR14/SENR. Recently, human homologues of both the receptor (UT-II) and the peptide (hU-II) have been discovered. Following de-orphanization, hU-II was declared the 'new endothelin' as initial studies suggested similarities between the peptides, and in isolated arteries of cynomolgus monkey U-II was a more potent constrictor than endothelin-1 (ET-1), with equal efficacy. However, effects of U-II in vascular tissue from other mammalian species are variable and although potent, U-II exhibits a lesser maximal response than ET-1. In contrast, in humans U-II has emerged as a ubiquitious constrictor of both arteries and veins in vitro and elicits a reduction in blood flow in the forearm and skin microcirculation in vivo. In addition to direct vasoconstrictor activity on smooth muscle receptors, endothelium-dependent U-II-mediated vasodilatation has also been observed. Non-vascular, peripheral actions of U-II include potent inotropy and airway smooth muscle constriction and U-II and its receptor are present throughout rat brain implying a possible neurotransmitter or neuromodulatory role in the central nervous system. U-II is proposed to contribute to human diseases including atherosclerosis, cardiac hypertrophy, pulmonary hypertension and tumour growth. The development of selective receptor antagonists should help to clarify the relative importance of hU-II as a multifunctional peptide in mammalian systems and its role in disease. What is clear is that U-II is emerging as a new and potentially important mammalian transmitter.
Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord.[Pubmed:9861051]
Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15803-8.
Urotensin II (UII) is a cyclic peptide initially isolated from the caudal neurosecretory system of teleost fish. Subsequently, UII has been characterized from a frog brain extract, indicating that a gene encoding a UII precursor is also present in the genome of a tetrapod. Here, we report the characterization of the cDNAs encoding frog and human UII precursors and the localization of the corresponding mRNAs. In both frog and human, the UII sequence is located at the C-terminal position of the precursor. Human UII is composed of only 11 amino acid residues, while fish and frog UII possess 12 and 13 amino acid residues, respectively. The cyclic region of UII, which is responsible for the biological activity of the peptide, has been fully conserved from fish to human. Northern blot and dot blot analysis revealed that UII precursor mRNAs are found predominantly in the frog and human spinal cord. In situ hybridization studies showed that the UII precursor gene is actively expressed in motoneurons. The present study demonstrates that UII, which has long been regarded as a peptide exclusively produced by the urophysis of teleost fish, is actually present in the brain of amphibians and mammals. The fact that evolutionary pressure has acted to conserve fully the biologically active sequence of UII suggests that the peptide may exert important physiological functions in humans.


