Fludarabine Phosphate (Fludara)Inhibits STAT1 activation and DNA synthesis CAS# 75607-67-9 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
Number of papers citing our products

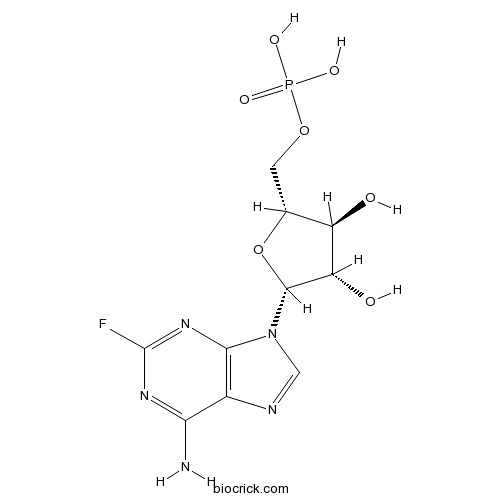
Chemical structure
3D structure
| Cas No. | 75607-67-9 | SDF | Download SDF |
| PubChem ID | 30751 | Appearance | Powder |
| Formula | C10H13FN5O7P | M.Wt | 365.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (273.82 mM) H2O : 5 mg/mL (13.69 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(2R,3S,4S,5R)-5-(6-amino-2-fluoropurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | ||
| SMILES | C1=NC2=C(N1C3C(C(C(O3)COP(=O)(O)O)O)O)N=C(N=C2N)F | ||
| Standard InChIKey | GIUYCYHIANZCFB-FJFJXFQQSA-N | ||
| Standard InChI | InChI=1S/C10H13FN5O7P/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,14,15)(H2,19,20,21)/t3-,5-,6+,9-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fludarabine is an inhibitor of STAT1 activation and a DNA synthesis. | |||||
| Targets | DNA synthesis | |||||

Fludarabine Phosphate (Fludara) Dilution Calculator

Fludarabine Phosphate (Fludara) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7382 mL | 13.6908 mL | 27.3815 mL | 54.763 mL | 68.4538 mL |
| 5 mM | 0.5476 mL | 2.7382 mL | 5.4763 mL | 10.9526 mL | 13.6908 mL |
| 10 mM | 0.2738 mL | 1.3691 mL | 2.7382 mL | 5.4763 mL | 6.8454 mL |
| 50 mM | 0.0548 mL | 0.2738 mL | 0.5476 mL | 1.0953 mL | 1.3691 mL |
| 100 mM | 0.0274 mL | 0.1369 mL | 0.2738 mL | 0.5476 mL | 0.6845 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fludarabine phosphate is a STAT-1 activation inhibitor and a DNA synthesis inhibitor. Fludarabine phosphate is the phosphate salt of a fluorinated nucleotide antimetabolite analog of the antiviral agent vidarabine (ara-A) with antineoplastic activity. Fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. This metabolite may inhibit DNA polymerase alpha, ribonucleotide reductase and DNA primase, thereby interrupting DNA synthesis and inhibiting tumor cell growth.
- Methyl 3,4,5-trimethoxycinnamate
Catalog No.:BCN4589
CAS No.:7560-49-8
- Kaerophyllin
Catalog No.:BCN4304
CAS No.:75590-33-9
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
- Sodium phosphate monobasic
Catalog No.:BCC8033
CAS No.:7558-80-7
- Sodium phosphate dibasic
Catalog No.:BCC7585
CAS No.:7558-79-4
- 20-Deoxyingenol 3-angelate
Catalog No.:BCN6642
CAS No.:75567-38-3
- Ingenol 3-Angelate
Catalog No.:BCN2961
CAS No.:75567-37-2
- Dencichin
Catalog No.:BCN2555
CAS No.:7554-90-7
- Moxonidine hydrochloride
Catalog No.:BCC5163
CAS No.:75536-04-8
- Nilvadipine
Catalog No.:BCC3799
CAS No.:75530-68-6
- Cedrin
Catalog No.:BCN4748
CAS No.:75513-81-4
- Flupirtine maleate
Catalog No.:BCC4456
CAS No.:75507-68-5
- (S)-(+)-α-Methylhistamine dihydrobromide
Catalog No.:BCC6700
CAS No.:75614-93-6
- (-)-Usnic acid
Catalog No.:BCN4306
CAS No.:7562-61-0
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
- Moracenin B
Catalog No.:BCC8341
CAS No.:75629-19-5
- Gomisin K1
Catalog No.:BCN7030
CAS No.:75629-20-8
- Oncrasin 1
Catalog No.:BCC2390
CAS No.:75629-57-1
- Knightolamine
Catalog No.:BCN1912
CAS No.:75638-70-9
- Chalcostrobamine
Catalog No.:BCN1900
CAS No.:75638-72-1
- DSLET
Catalog No.:BCC5718
CAS No.:75644-90-5
- Boc-D-Asn-OH
Catalog No.:BCC3362
CAS No.:75647-01-7
- Strobamine
Catalog No.:BCN1943
CAS No.:75656-91-6
- 4-Acetyl-1,1-dimethylpiperazinium iodide
Catalog No.:BCC6616
CAS No.:75667-84-4
[The use of fludarabine phosphate (Fludara) to treat chronic B-cell lymphocytic leukemia and non-Hodgkin's lymphoma resistant to standard chemotherapy].[Pubmed:10087967]
Vopr Onkol. 1998;44(6):696-700.
Following monochemotherapy with fludarabin phosphate (fludara), complete (18%), and partial remission (27%) with stabilization (45%) was recorded in patients with B-cell chronic lymphocytic leukemia, most of whom were resistant to alkylating cytostatic drugs and their combinations containing anthracyclines. Long-term remission was registered in patients with low and moderate grade non-Hodgkin's lymphoma, resistant to standard cytostatic treatment, due to the therapeutic synergism of fludara and cytosinarabinoside (FAVAMP).


