Taccabulin ACAS# 1464719-37-6 |
Quality Control & MSDS
Number of papers citing our products

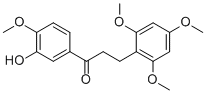
Chemical structure

| Cas No. | 1464719-37-6 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C19H22O6 | M.Wt | 346.41 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Taccabulin A Dilution Calculator

Taccabulin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8868 mL | 14.4338 mL | 28.8675 mL | 57.7351 mL | 72.1688 mL |
| 5 mM | 0.5774 mL | 2.8868 mL | 5.7735 mL | 11.547 mL | 14.4338 mL |
| 10 mM | 0.2887 mL | 1.4434 mL | 2.8868 mL | 5.7735 mL | 7.2169 mL |
| 50 mM | 0.0577 mL | 0.2887 mL | 0.5774 mL | 1.1547 mL | 1.4434 mL |
| 100 mM | 0.0289 mL | 0.1443 mL | 0.2887 mL | 0.5774 mL | 0.7217 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Senarguine A
Catalog No.:BCX0598
CAS No.:1174182-45-6
- 5,7,3',4',5'-Pentahydroxyflavan
Catalog No.:BCX0597
CAS No.:493-44-7
- Stelleranoid B
Catalog No.:BCX0596
CAS No.:2957870-90-3
- Wikstrol B
Catalog No.:BCX0595
CAS No.:160963-92-8
- Cyclocarioside K
Catalog No.:BCX0594
CAS No.:2093058-16-1
- Genkwanol A
Catalog No.:BCX0593
CAS No.:111103-90-3
- Noueloside B
Catalog No.:BCX0592
CAS No.:2172630-88-3
- 7,2',4'-Trihydroxyflavanone
Catalog No.:BCX0591
CAS No.:128837-33-2
- Thujaplicatin
Catalog No.:BCX0590
CAS No.:6512-66-9
- ent-11α,12α,15α-Trihydroxykaur-16-en-19-oic acid
Catalog No.:BCX0589
CAS No.:57719-84-3
- Cyclocarioside I
Catalog No.:BCX0588
CAS No.:1644624-82-7
- 6'-O-Acetyldaucosterol
Catalog No.:BCX0587
CAS No.:870093-75-7
- Taccabulin A methyl ether
Catalog No.:BCX0600
CAS No.:42924-06-1
- Maximowicziol A
Catalog No.:BCX0601
CAS No.:193153-41-2
- Plantagiolide A
Catalog No.:BCX0602
CAS No.:913263-83-9
- Plantagiolide B
Catalog No.:BCX0603
CAS No.:913263-85-1
- Graveolone
Catalog No.:BCX0604
CAS No.:16499-05-1
- Urolithin B
Catalog No.:BCX0605
CAS No.:1139-83-9
- Prosapogenin F
Catalog No.:BCX0606
CAS No.:99365-20-5
- Epipinoresinol-4'-O-glucopyranoside
Catalog No.:BCX0607
CAS No.:74983-66-7
- 5’-inosinic acid
Catalog No.:BCX0608
CAS No.:131-99-7
- Arundamine
Catalog No.:BCX0609
CAS No.:475977-53-8
- Stugeron
Catalog No.:BCX0610
CAS No.:298-57-7
- OJV-VI
Catalog No.:BCX0611
CAS No.:125150-67-6
Structure-activity relationships of retro-dihydrochalcones isolated from Tacca sp.[Pubmed:24303844]
J Nat Prod. 2013 Dec 27;76(12):2189-94.
Several biologically active compounds have been identified from Tacca species, including glycosides, diarylheptanoids, saponins, withanolides, and the taccalonolide class of microtubule stabilizers. More recently, two cytotoxic retro-dihydrochalcones named evelynin A (7) and Taccabulin A (6) were isolated and their biological activities characterized, including the finding that taccabulin has microtubule destabilizing effects. Here we describe the identification and characterization of five new retro-chalcones, named taccabulins B-E (1-4) and evelynin B (5) from Tacca sp. extracts. Their structures were determined using 1D and 2D NMR as well as mass spectroscopic data and modeled into the colchicine binding site of tubulin. The antiproliferative and microtubule effects of each compound were determined experimentally and found to be well correlated with modeling studies. The isolation and biological characterization of several retro-dihydrochalcones facilitated preliminary structure-activity relationships for this compound class concerning its antiproliferative and microtubule depolymerizing activities.
The bat flower: a source of microtubule-destabilizing and -stabilizing compounds with synergistic antiproliferative actions.[Pubmed:24087857]
J Nat Prod. 2013 Oct 25;76(10):1923-9.
The biosynthesis of secondary metabolites provides higher plants with mechanisms of defense against microbes, insects, and herbivores. One common cellular target of these molecules is the highly conserved microtubule cytoskeleton, and microtubule-targeting compounds with insecticidal, antifungal, nematicidal, and anticancer activities have been identified from plants. A new retro-dihydrochalcone, Taccabulin A, with microtubule-destabilizing activity has been identified from the roots and rhizomes of Tacca species. This finding is notable because the microtubule-stabilizing taccalonolides are also isolated from these sources. This is the first report of an organism producing compounds with both microtubule-stabilizing and -destabilizing activities. A two-step chemical synthesis of Taccabulin A was performed. Mechanistic studies showed that Taccabulin A binds within the colchicine site on tubulin and has synergistic antiproliferative effects against cancer cells when combined with a taccalonolide, which binds to a different site on tubulin. Taccabulin A is effective in cells that are resistant to many other plant-derived compounds. The discovery of a natural source that contains both microtubule-stabilizing and -destabilizing small molecules is unprecedented and suggests that the synergistic action of these compounds was exploited by nature long before it was discovered in the laboratory.


