Genkwanol ACAS# 111103-90-3 |
Quality Control & MSDS
Number of papers citing our products

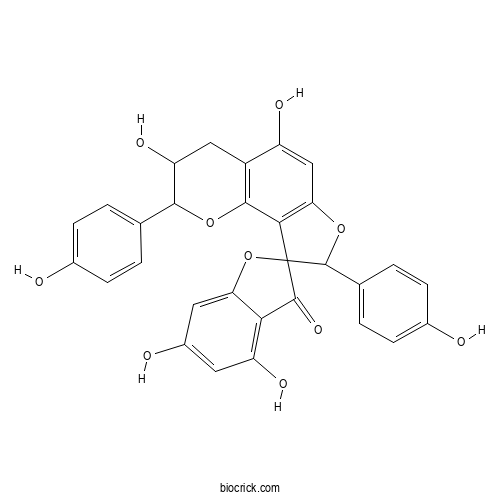
Chemical structure

3D structure
| Cas No. | 111103-90-3 | SDF | Download SDF |
| PubChem ID | 500379 | Appearance | Powder |
| Formula | C30H22O10 | M.Wt | 542.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3',4,5',6-tetrahydroxy-2',8'-bis(4-hydroxyphenyl)spiro[1-benzofuran-2,9'-2,3,4,8-tetrahydrofuro[2,3-h]chromene]-3-one | ||
| SMILES | C1C(C(OC2=C1C(=CC3=C2C4(C(O3)C5=CC=C(C=C5)O)C(=O)C6=C(C=C(C=C6O4)O)O)O)C7=CC=C(C=C7)O)O | ||
| Standard InChIKey | BYBKYSAHKVMKNH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H22O10/c31-15-5-1-13(2-6-15)26-21(36)11-18-19(34)12-23-25(27(18)39-26)30(29(38-23)14-3-7-16(32)8-4-14)28(37)24-20(35)9-17(33)10-22(24)40-30/h1-10,12,21,26,29,31-36H,11H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Genkwanol A Dilution Calculator

Genkwanol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8433 mL | 9.2166 mL | 18.4332 mL | 36.8664 mL | 46.0829 mL |
| 5 mM | 0.3687 mL | 1.8433 mL | 3.6866 mL | 7.3733 mL | 9.2166 mL |
| 10 mM | 0.1843 mL | 0.9217 mL | 1.8433 mL | 3.6866 mL | 4.6083 mL |
| 50 mM | 0.0369 mL | 0.1843 mL | 0.3687 mL | 0.7373 mL | 0.9217 mL |
| 100 mM | 0.0184 mL | 0.0922 mL | 0.1843 mL | 0.3687 mL | 0.4608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Noueloside B
Catalog No.:BCX0592
CAS No.:2172630-88-3
- 7,2',4'-Trihydroxyflavanone
Catalog No.:BCX0591
CAS No.:128837-33-2
- Thujaplicatin
Catalog No.:BCX0590
CAS No.:6512-66-9
- ent-11α,12α,15α-Trihydroxykaur-16-en-19-oic acid
Catalog No.:BCX0589
CAS No.:57719-84-3
- Cyclocarioside I
Catalog No.:BCX0588
CAS No.:1644624-82-7
- 6'-O-Acetyldaucosterol
Catalog No.:BCX0587
CAS No.:870093-75-7
- Laurotetanine
Catalog No.:BCX0586
CAS No.:128-76-7
- Beauvericin A
Catalog No.:BCX0585
CAS No.:165467-50-5
- Bassianolide
Catalog No.:BCX0584
CAS No.:64763-82-2
- Glochidionionoside D
Catalog No.:BCX0583
CAS No.:620598-43-8
- Hydrangeifolin I
Catalog No.:BCX0582
CAS No.:88510-10-5
- 9-Oxoailanthoidol
Catalog No.:BCX0581
CAS No.:912280-23-0
- Cyclocarioside K
Catalog No.:BCX0594
CAS No.:2093058-16-1
- Wikstrol B
Catalog No.:BCX0595
CAS No.:160963-92-8
- Stelleranoid B
Catalog No.:BCX0596
CAS No.:2957870-90-3
- 5,7,3',4',5'-Pentahydroxyflavan
Catalog No.:BCX0597
CAS No.:493-44-7
- Senarguine A
Catalog No.:BCX0598
CAS No.:1174182-45-6
- Taccabulin A
Catalog No.:BCX0599
CAS No.:1464719-37-6
- Taccabulin A methyl ether
Catalog No.:BCX0600
CAS No.:42924-06-1
- Maximowicziol A
Catalog No.:BCX0601
CAS No.:193153-41-2
- Plantagiolide A
Catalog No.:BCX0602
CAS No.:913263-83-9
- Plantagiolide B
Catalog No.:BCX0603
CAS No.:913263-85-1
- Graveolone
Catalog No.:BCX0604
CAS No.:16499-05-1
- Urolithin B
Catalog No.:BCX0605
CAS No.:1139-83-9
Effects of an ethyl acetate extract of Daphne altaica stem bark on the cell cycle, apoptosis and expression of PPARgamma in Eca‑109 human esophageal carcinoma cells.[Pubmed:32468007]
Mol Med Rep. 2020 Aug;22(2):1400-1408.
Daphne altaica Pall. (D. altaica; Thymelaeaceae) has long been used in traditional Kazakh medicine for the treatment of cancer and respiratory diseases. Previous studies have demonstrated the in vitro anticancer effects of D. altaica extract and its constituents in certain cancer cell lines; however, the underlying molecular mechanisms are not completely understooD. The present study aimed to investigate the molecular mechanisms underlying the activity of an ethyl acetate extract of D. altaica (Da‑Ea) by assessing its effects on cell morphology, cell apoptosis, cell cycle progression and the expression levels of peroxisome proliferator‑activated receptor gamma (PPARgamma) in Eca‑109 cells. Cell morphology was observed under a phase contrast microscope. Cell apoptosis and cell cycle progression were assessed by flow cytometry following Annexin V/propidium iodide (PI) double staining and PI single staining, respectively. The mRNA and protein expression levels of PPARgamma were determined by reverse transcription‑quantitative PCR and western blotting, respectively. Compared with the control group, the percentage of apoptotic cells, cell cycle arrest at S phase and apoptotic morphological cell characteristics were increased in Da‑Ea‑treated Eca‑109 cells. Furthermore, Da‑Ea treatment upregulated the mRNA and protein expression levels of PPARgamma compared with the control cells. High‑performance liquid chromatography with diode‑array detection indicated that daphnetin‑7‑O‑beta‑D‑glucoside, daphnetin, demethyldaphnoretin‑7‑O‑beta‑D‑glucopyranoside and Genkwanol A were the main constituents of Da‑Ea. Collectively, the results suggested that Da‑Ea displayed antiproliferative activities in Eca‑109 cells by inducing apoptosis and S phase cell cycle arrest, as well as upregulating PPARgamma expression levels.
A new sesquiterpenoid from the shoots of Iranian Daphne mucronata Royle with selective inhibition of STAT3 and Smad3/4 cancer-related signaling pathways.[Pubmed:32248516]
Daru. 2020 Jun;28(1):253-262.
PURPOSE: Daphne mucronata Royle grown in Iran has shown anticancer activities against different cancer cell lines. Therefore, within this study, we investigate the phytochemical pattern of this plant. METHOD: Phytochemical investigation was done using standard column chromatography system: The structures were recognized by the interpretation of one and two-dimensional nuclear magnetic resonance (NMR) spectra and the help of High-Resolution Electrospray Ionization Mass spectroscopy (HR-ESIMS) and Infrared spectroscopy (IR) data. Stereochemistry was determined using 2D and 3D NOESY, and comparison of coupling constant values with literature. The absolute configuration was determined and confirmed using specific rotation and electronic circular dichroism experiments. Cytotoxicity was done against HeLa cells by standard MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Luciferase assay was used to check if the compounds can inhibit the activation of cancer-related signaling pathways. Molecular docking simulation was done for biological activity evaluation and to examine the interaction of the ligand with each of the proteins. RESULTS: A new sesquiterpenoid, 4,11(12)-guiadiene-1-ol-3-one (4), together with eight specialized metabolites, betulinic acid (1), coniferyl aldehyde (2), oleanolic acid (3), daphnetoxin (5), apigenin (7), syringin (8), and Genkwanol A (9) were isolated and reported for the first time from the shoots of the plant. Compound 4 as an undescribed compound was submitted for cytotoxicity assay and showed moderate activity with the IC(50) value of 51.3 +/- 4.2 muM against HeLa cancer cells. It showed selective inhibition of Interleukin-6 mediated signal transducer and activator of transcription 3 pathway (STAT-3/ IL-6), and Smad protein / transforming growth factor beta (TGF-beta) transcription factors when screened through an array of cancer signaling pathways. Molecular docking confirmed biological tests and showed the interaction with STAT3 and Smad proteins. CONCLUSION: An undescribed sesquiterpenoid: 4,11(12)-guiadiene-1-ol-3-one in addition to eight known compounds were isolated. The new sesquiterpene was evaluated for the luciferase assay on 14 main cancer-related signaling pathways and showed selective inhibition of STAT3/IL6, and Smad/ TGF-beta transcription factors. Molecular docking simulation showed more interactions with STAT3 than Smad, which confirms better interaction of compound 4 with STAT3 than Smad proteins. Graphical abstract.
Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae).[Pubmed:25096753]
Phytochemistry. 2014 Oct;106:61-68.
Allelopathy, the negative effect on plants of chemicals released to the surroundings by a neighboring plant, is an important factor which contributes to the spread of some weeds in plant communities. In this field, Stellera chamaejasme L. (Thymelaeaceae) is one of the most toxic and ecologically-threatening weeds in some of the grasslands of north and west China. Bioassay-guided fractionation of root extracts of this plant led to the isolation of eight flavonoids 1-8, whose structures were elucidated by spectroscopic analysis. All compounds obtained, except 7-methoxylneochaejasmin A (4) and (+)-epiafzelechin (5), showed strong phytotoxic activity against Arabidopsis thaliana seedlings. Seedling growth was reduced by neochamaejasmin B (1), mesoneochamaejasmin A (2), chamaejasmenin C (3), Genkwanol A (6), daphnodorin B (7) and dihydrodaphnodorin B (8) with IC50 values of 6.9, 12.1, 43.2, 74.8, 7.1 and 27.3mug/mL, respectively, and all of these compounds disrupted root development. Endogenous auxin levels at the root tips of the A. thaliana DR5::GUS transgenic line were largely reduced by compounds 1, 2 and 6-8, and were increased by compound 4. Moreover, the inhibition rate of A. thaliana auxin transport mutants pin2 and aux1-7 by compounds 1-8 were all lower than the wild type (Col-0). The influence of these compounds on endogenous auxin distribution is thus proposed as a critical factor for the phytotoxic effect. Compounds 1, 2, 4 and 8 were found in soils associated with S. chamaejasme, and these flavonoids also showed phytotoxicity to Clinelymus nutans L., an associated weed of S. chamaejasme. These results indicated that some phytotoxic compounds from roots of S. chamaejasme may be involved in the potential allelopathic behavior of this widespread weed.
Microphynolides A and B, new spiro-gamma-lactone glycosides from Thymelaea microphylla.[Pubmed:25076123]
Nat Prod Res. 2014;28(20):1732-8.
Two new spiro-gamma-lactone glycosides named microphynolide A (1) and microphynolide B (2), together with twelve known compounds including five biflavonoids namely neochamaejasmin A, neochamaejasmin B, daphnodorin B, Genkwanol A and stelleranol, one bis-coumarin daphnoretin, two lignans called pinoresinol and matairesinol, one flavonoid glucoside, tiliroside, a sinapyl alcohol glucoside, syringin, and two phytosterols, beta-sitosterol and beta-sitosterol-3-O-glucoside, were isolated from ethyl acetate extracts of the aerial parts and roots of the plant Thymelaea microphylla Coss. and Dur. All the isolated compounds were characterised by using spectroscopic methods and comparison with the literature data.
Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica.[Pubmed:10985087]
Planta Med. 2000 Aug;66(6):564-7.
With guidance of Pyricularia oryzae bioassay, daphnoretin (1), (+)-nortrachelogenin (2), Genkwanol A (3), wikstrol A (4), wikstrol B (5) and daphnodorin B (6) were isolated from the roots of Wikstroemia indica. Compounds 1-6 induced morphological deformation of P. oryzae mycelia with MMDC values of 68.4 +/- 1.3, 31.3 +/- 1.8, 45.8 +/- 0.5, 70.1 +/- 2.4, 52.3 +/- 0.9 and 73.7 +/- 1.6 microM, respectively. Compounds 3-6 showed moderate activity against microtubule polymerization with IC50 values of 112 +/- 4, 131 +/- 3, 184 +/- 6 and 142 +/- 2 microM in vitro, respectively. Compounds 2, 3, 5 and 6 were moderately active against HIV-1 in vitro. The findings of bioactivity of 1-6 support the antifungus, antimitosis and anti-HIV-1 uses for W. indica roots.


