GraveoloneCAS# 16499-05-1 |
Quality Control & MSDS
Number of papers citing our products

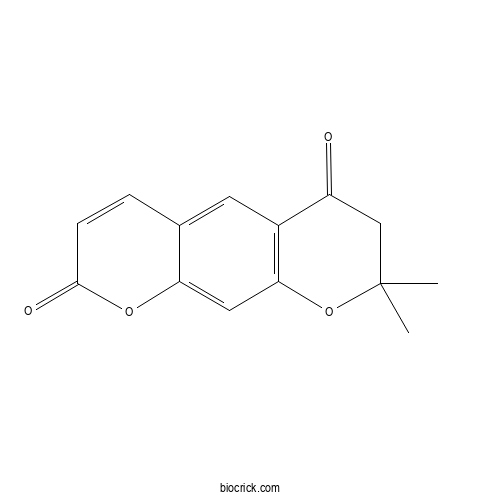
Chemical structure

3D structure
| Cas No. | 16499-05-1 | SDF | Download SDF |
| PubChem ID | 177751 | Appearance | Powder |
| Formula | C14H12O4 | M.Wt | 244.24 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,2-dimethyl-3H-pyrano[3,2-g]chromene-4,8-dione | ||
| SMILES | CC1(CC(=O)C2=C(O1)C=C3C(=C2)C=CC(=O)O3)C | ||
| Standard InChIKey | WEDGVCZUPFZNDE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12O4/c1-14(2)7-10(15)9-5-8-3-4-13(16)17-11(8)6-12(9)18-14/h3-6H,7H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Graveolone Dilution Calculator

Graveolone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0943 mL | 20.4717 mL | 40.9433 mL | 81.8867 mL | 102.3583 mL |
| 5 mM | 0.8189 mL | 4.0943 mL | 8.1887 mL | 16.3773 mL | 20.4717 mL |
| 10 mM | 0.4094 mL | 2.0472 mL | 4.0943 mL | 8.1887 mL | 10.2358 mL |
| 50 mM | 0.0819 mL | 0.4094 mL | 0.8189 mL | 1.6377 mL | 2.0472 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4094 mL | 0.8189 mL | 1.0236 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Plantagiolide B
Catalog No.:BCX0603
CAS No.:913263-85-1
- Plantagiolide A
Catalog No.:BCX0602
CAS No.:913263-83-9
- Maximowicziol A
Catalog No.:BCX0601
CAS No.:193153-41-2
- Taccabulin A methyl ether
Catalog No.:BCX0600
CAS No.:42924-06-1
- Taccabulin A
Catalog No.:BCX0599
CAS No.:1464719-37-6
- Senarguine A
Catalog No.:BCX0598
CAS No.:1174182-45-6
- 5,7,3',4',5'-Pentahydroxyflavan
Catalog No.:BCX0597
CAS No.:493-44-7
- Stelleranoid B
Catalog No.:BCX0596
CAS No.:2957870-90-3
- Wikstrol B
Catalog No.:BCX0595
CAS No.:160963-92-8
- Cyclocarioside K
Catalog No.:BCX0594
CAS No.:2093058-16-1
- Genkwanol A
Catalog No.:BCX0593
CAS No.:111103-90-3
- Noueloside B
Catalog No.:BCX0592
CAS No.:2172630-88-3
- Urolithin B
Catalog No.:BCX0605
CAS No.:1139-83-9
- Prosapogenin F
Catalog No.:BCX0606
CAS No.:99365-20-5
- Epipinoresinol-4'-O-glucopyranoside
Catalog No.:BCX0607
CAS No.:74983-66-7
- 5’-inosinic acid
Catalog No.:BCX0608
CAS No.:131-99-7
- Arundamine
Catalog No.:BCX0609
CAS No.:475977-53-8
- Stugeron
Catalog No.:BCX0610
CAS No.:298-57-7
- OJV-VI
Catalog No.:BCX0611
CAS No.:125150-67-6
- Oleanic aldehyde
Catalog No.:BCX0612
CAS No.:17020-22-3
- 3,5-Dimethoxytoluene
Catalog No.:BCX0613
CAS No.:4179-19-5
- Resveratrol-4'-O-β-D-(6''-O-galloy)-glucopyranoside
Catalog No.:BCX0614
CAS No.:64898-03-9
- 3,4,5-Trimethoxytoluene
Catalog No.:BCX0615
CAS No.:6443-69-2
- 3’,5-dihydroxy-2-(4-hydroxybenzyl)3-methoxybibenzyl
Catalog No.:BCX0616
CAS No.:151538-57-7
Isolation and in silico prediction of potential drug-like compounds from Anethum sowa L. root extracts targeted towards cancer therapy.[Pubmed:30584950]
Comput Biol Chem. 2019 Feb;78:242-259.
Anethum sowa L. has been used as a spice herb in the Asian and European culinary systems to add flavour and taste. The studied plant has diverse folkloric medicinal value. Present study was designed to isolate phytochemicals from the hexane, chloroform and ethyl acetate extracts of the roots by various chromatographic techniques. Based on spectral analysis (IR, LC-MS, NMR) the isolated compounds were identified as physcione (1), beta-sitosterol (2), stigmasterol (3), 2-oxo-3-propyl-2H-chromene-7-carboxylic acid (4), bergapten (5), 3-ethyl-7-hydroxy-2H-chromen-2-one (6) and Graveolone (7). The mentioned compounds have been isolated for the first time from the roots part of the plant. Based on extensive literature review, physcione and bergapten were inferred to exhibit crucial bioactivities including inhibitory efficacy against various forms of cancer. Accordingly, in the present research approach molecular docking investigations of the isolated phytochemicals have been robustly executed with different oncogenes that have been reported to be actively involved in various forms of carcinoma. In silico investigations encompassing molecular docking analysis and drug-likeness profiling was executed to estimate the potential therapeutic tendencies of the phytochemicals targeted towards effective cancer therapy. Current investigation offers meaningful know-how pertaining to potential anticancer activities of the phytochemicals extracted from the roots of Anethum sowa L. and might open up new revenues towards effective drug development against cancer.
[Chemical constituents from branch of Broussonetia papyrifera].[Pubmed:17048607]
Zhongguo Zhong Yao Za Zhi. 2006 Jul;31(13):1078-80.
OBJECTIVE: To investigate the chemical constituents from the branch of Broussonetia papyrifera. METHOD: Column chromatographic methods were used to isolate the chemical constituents. ESI-MS and NMR methods were employed for their structural elucidation. RESULT: Six compounds were isolated and identified as (2S)-7, 3'-dihydroxy-4'-methoxyflavan (1), ergosterol peroxide (2), D-galacitol (3), sulfuretin (4), liriodendrin (5), Graveolone (6), respectively. CONCLUSION: Compounds 1-6 were isolated from the plant for the first time.
Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 2. Effects on enzyme activities.[Pubmed:6682039]
Eur J Biochem. 1983 Mar 15;131(2):409-13.
Parsley cell cultures produce linear furanocoumarins and the linear benzodipyrandione, Graveolone, in response to treatment with an elicitor from either Phytophthora megasperma or Alternaria carthami. Activities of enzymes involved in general phenylpropanoid metabolism, phenylalanine ammonia-lyase and 4-coumarate: CoA ligase, as well as of an enzyme involved specifically in furanocoumarin biosynthesis, dimethylallyl diphosphate: umbelliferone dimethylallyltransferase, were monitored over several days after treatment with A. carthami elicitor. In addition, the activities of chalcone synthase, an enzyme involved in flavonoid formation, and of glucose-6-phosphate: NADP 1-oxidoreductase were also monitored. The lyase and the ligase activities increased steadily for 48 h and the dimethylallyltransferase activity for 54 h, while the synthase activity was not altered and the oxidoreductase activity decreased gradually. In some experiments, phenylalanine ammonia-lyase activity reached a maximum value of 250 mukat/kg, twice the maximal activity observed previously in parsley cells after treatment with either ultraviolet light or an elicitor preparation from P. megasperma. In crude extracts, phenylalanine ammonia-lyase activity was shown to be inhibited by unidentified small-molecular-weight compounds which were formed in proportion to the elicitor treatment. While phenylalanine ammonia-lyase and dimethylallyl diphosphate: umbelliferone dimethylallyltransferase are known to be required for furanocoumarin biosynthesis, the involvement of 4-coumarate: CoA ligase is as yet unclear. The concomitant increase and decrease of the ligase activity with the activities of the lyase and the dimethylallyltransferase, as well as its similar response to elicitor concentrations, suggest that CoA esters of cinnamic acids play a role in the biosynthesis of furanocoumarins.
Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives.[Pubmed:6682038]
Eur J Biochem. 1983 Mar 15;131(2):401-7.
Dark-grown cell suspension cultures of parsley, Petroselinum hortense, produce furanocoumarins after treatment with elicitor preparations of either Phytophthora megasperma f.sp. glycinea (Pmg elicitor) or Alternaria carthami Chowdhury (Ac elicitor). The linear furanocoumarins, psoralen and xanthotoxin, and the benzodipyrandione, Graveolone, are the major products synthesized in response to Pmg elicitor, besides small amounts of the furanocoumarin bergapten. Treatment with Ac elicitor induces predominantly the formation of bergapten and the furanocoumarin isopimpinellin, as well as small amounts of Graveolone. While Pmg elicitor leads to cell death within a few days, cell mass increased for at least 6 days after treatment with Ac elicitor. Brefeldin A, a phytotoxin produced by A. carthami, inhibits growth of parsley cell suspension cultures considerably at a concentration of 0.01 mM and growth of the cells ceased at a concentration of 0.1 mM toxin. Concomitantly, furanocoumarin biosynthesis was suppressed in our system by a concentration of brefeldin A within 0.01-0.1 mM.


