ColchicineTubulin Inhibitor CAS# 64-86-8 |
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Eribulin mesylate
Catalog No.:BCC5173
CAS No.:441045-17-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 64-86-8 | SDF | Download SDF |
| PubChem ID | 6167 | Appearance | White-pale yellow powder |
| Formula | C22H25NO6 | M.Wt | 399.44 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 48 mg/mL (120.17 mM) H2O : ≥ 33.33 mg/mL (83.44 mM) *"≥" means soluble, but saturation unknown. | ||
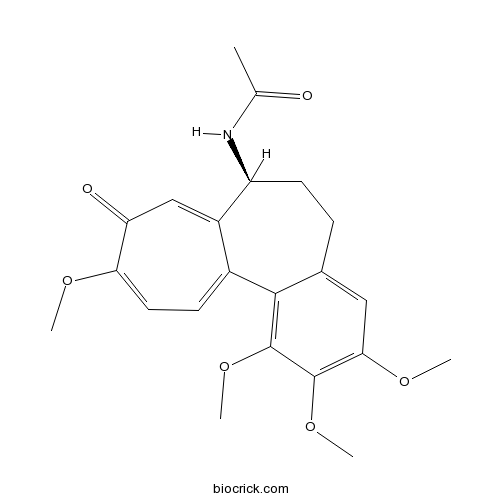
| Chemical Name | N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide | ||
| SMILES | CC(=O)NC1CCC2=CC(=C(C(=C2C3=CC=C(C(=O)C=C13)OC)OC)OC)OC | ||
| Standard InChIKey | IAKHMKGGTNLKSZ-INIZCTEOSA-N | ||
| Standard InChI | InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Colchicine is a tubulin inhibitor, and a microtubule polymerization inhibitor with an IC50 of 3 nM. It prevents amyloidosis in our high-risk population and that it can prevent additional deterioration of renal function in patients with amyloidosis who have proteinuria but not the nephrotic syndrome. Colchicine has anti-mitotic activity, it can be used to treat familial mediterranean fever, and as a lead compound for the generation of potent anti-cancer drugs. |
| Targets | IL Receptor | TNF-α |
| In vitro | Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin.[Pubmed: 17464966 ]Med Res Rev. 2008 Jan;28(1):155-83.In this review, an attempt has been made to throw light on the mechanism of action of Colchicine and its different analogs as anti-cancer agents. Colchicine interacts with tubulin and perturbs the assembly dynamics of microtubules. Though its use has been limited because of its toxicity, Colchicine can still be used as a lead compound for the generation of potent anti-cancer drugs. Colchicine binds to tubulin in a poorly reversible manner with high activation energy. The binding interaction is favored entropically. In contrast, binding of its simple analogs AC or DAAC is enthalpically favored and commences with comparatively low activation energy. Colchicine-tubulin interaction, which is normally pH dependent, has been found to be independent of pH in the presence of microtubule-associated proteins, salts or upon cleavage of carboxy termini of tubulin. Biphasic kinetics of Colchicines-tubulin interaction has been explained in light of the variation in the residues around the drug-binding site on beta-tubulin. Using the crystal structure of the tubulin-DAMAColchicine complex, a detailed discussion on the pharmacophore concept that explains the variation of affinity for different Colchicine site inhibitors (CSI) has been discussed. |
| In vivo | Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever.[Pubmed: 3515182 ]N Engl J Med. 1986 Apr 17;314(16):1001-5.
Colchicine Therapy for Familial Mediterranean Fever: A Double-Blind Trial[Reference: WebLink]New Engl. J. Med., 1974, 291(18): 934-7.
|
| Cell Research | Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils.[Pubmed: 7543498 ]J Clin Invest. 1995 Aug;96(2):994-1002.
|
| Structure Identification | Nature, 2004, 428(6979):198-202.Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain.[Pubmed: 15014504]Microtubules are cytoskeletal polymers of tubulin involved in many cellular functions. Their dynamic instability is controlled by numerous compounds and proteins, including Colchicine and stathmin family proteins. The way in which microtubule instability is regulated at the molecular level has remained elusive, mainly because of the lack of appropriate structural data.
|

Colchicine Dilution Calculator

Colchicine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5035 mL | 12.5175 mL | 25.035 mL | 50.0701 mL | 62.5876 mL |
| 5 mM | 0.5007 mL | 2.5035 mL | 5.007 mL | 10.014 mL | 12.5175 mL |
| 10 mM | 0.2504 mL | 1.2518 mL | 2.5035 mL | 5.007 mL | 6.2588 mL |
| 50 mM | 0.0501 mL | 0.2504 mL | 0.5007 mL | 1.0014 mL | 1.2518 mL |
| 100 mM | 0.025 mL | 0.1252 mL | 0.2504 mL | 0.5007 mL | 0.6259 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Colchicine, a tubulin inhibitor, which block polymerization of microtubules by binding to tubulin (IC50 = 3.2 μM).
Tubulin is one of several members of a small family of globular proteins. The tubulin superfamily includes five distinct families. To form microtubules, the dimers of α- and β-tubulin bind to GTP and assemble onto the (+) ends of microtubules while in the GTP-bound state.[1] The β-tubulin subunit is exposed on the plus end of the microtubule while the α-tubulin subunit is exposed on the minus end. After the dimer is incorporated into the microtubule, the molecule of GTP bound to the β-tubulin subunit eventually hydrolyzes into GDP through inter-dimer contacts along the microtubule protofilament.[2] This is the GTP cycle which is essential for the dynamic instability of the microtubule.
Colchicine inhibits microtubule polymerization by binding to tubulin, one of the main constituents of microtubules. Availability of tubulin is essential to mitosis, and therefore colchicine effectively functions as a "mitotic poison" or spindle poison.[3] So mitosis can be stopped before it completes near the middle of mitosis (specifically metaphase) in the cell cycle. Apart from inhibiting mitosis, colchicine also inhibits neutrophil motility and activity, leading to a net anti-inflammatory effect in 5 μmol/kg in a mouse model of gouty arthritis and inhibits the deposition of uric acid, a key aspect in the treatment of gout.[4] Side-effects include gastrointestinal upset and neutropenia. High doses can also damage bone marrow and lead to anemia and also cause hair loss.[5]
References:
1. Heald R, Nogales E. "Microtubule dynamics". J. Cell. Sci. 2002,115 (Pt 1): 3–4.
2. Howard J, Hyman A. "Dynamics and mechanics of the microtubule plus end". Nature 2003,422 (6933): 753–8.
3. "Information for Healthcare Professionals: New Safety Information for Colchicine (marketed as Colcrys)". U.S. Food and Drug Administration.
4. Chen LX, Schumacher HR. "Gout: an evidence-based review". J Clin Rheumatol 2008, 14: S55–62.
5. Colchicine. National Institute for Occupational Safety and Health. Emergency Response Safety and Health Database, August 22, 2008. Retrieved December 23, 2008.
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Tetracycline Hydrochloride
Catalog No.:BCC1206
CAS No.:64-75-5
- Demeclocycline hydrochloride
Catalog No.:BCC5303
CAS No.:64-73-3
- Physostigmine hemisulfate
Catalog No.:BCC6724
CAS No.:64-47-1
- Lobeline Hydrochloride
Catalog No.:BCC8202
CAS No.:63990-84-1
- 13-Oxopodocarp-8(14)-en-18-oic acid
Catalog No.:BCN4009
CAS No.:63976-69-2
- Hopeyhopin
Catalog No.:BCN7533
CAS No.:63975-56-4
- Artemisinin
Catalog No.:BCN5814
CAS No.:63968-64-9
- (3R,10S)-Heptadeca-1,8-diene-4,6-diyne-3,10-diol
Catalog No.:BCC9111
CAS No.:63910-76-9
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- VUF 5681 dihydrobromide
Catalog No.:BCC7383
CAS No.:639089-06-8
- LH846
Catalog No.:BCC4246
CAS No.:639052-78-1
- Etimizol
Catalog No.:BCC1562
CAS No.:64-99-3
- H-D-Val-OH
Catalog No.:BCC3145
CAS No.:640-68-6
- Paroxetine maleate
Catalog No.:BCC7265
CAS No.:64006-44-6
- Tamgermanetin
Catalog No.:BCN7944
CAS No.:640235-90-1
- 4(15)-Oppositene-1,7-diol
Catalog No.:BCN4180
CAS No.:640289-58-3
- Torachrysone 8-O-glucoside
Catalog No.:BCN4181
CAS No.:64032-49-1
- Boc-Lys(Ac)-OH
Catalog No.:BCC3411
CAS No.:6404-26-8
- Boc-Nle-OH
Catalog No.:BCC3296
CAS No.:6404-28-0
- Boc-ε-Acp-OH
Catalog No.:BCC3205
CAS No.:6404-29-1
- Z-D-Pro-OH
Catalog No.:BCC2752
CAS No.:6404-31-5
- Ro 04-5595 hydrochloride
Catalog No.:BCC7234
CAS No.:64047-73-0
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin.[Pubmed:17464966]
Med Res Rev. 2008 Jan;28(1):155-83.
In this review, an attempt has been made to throw light on the mechanism of action of Colchicine and its different analogs as anti-cancer agents. Colchicine interacts with tubulin and perturbs the assembly dynamics of microtubules. Though its use has been limited because of its toxicity, Colchicine can still be used as a lead compound for the generation of potent anti-cancer drugs. Colchicine binds to tubulin in a poorly reversible manner with high activation energy. The binding interaction is favored entropically. In contrast, binding of its simple analogs AC or DAAC is enthalpically favored and commences with comparatively low activation energy. Colchicine-tubulin interaction, which is normally pH dependent, has been found to be independent of pH in the presence of microtubule-associated proteins, salts or upon cleavage of carboxy termini of tubulin. Biphasic kinetics of Colchicines-tubulin interaction has been explained in light of the variation in the residues around the drug-binding site on beta-tubulin. Using the crystal structure of the tubulin-DAMAColchicine complex, a detailed discussion on the pharmacophore concept that explains the variation of affinity for different Colchicine site inhibitors (CSI) has been discussed.
Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever.[Pubmed:3515182]
N Engl J Med. 1986 Apr 17;314(16):1001-5.
To determine whether Colchicine prevents or ameliorates amyloidosis in patients with familial Mediterranean fever, we followed 1070 patients with the latter disease for 4 to 11 years after they were advised to take Colchicine to prevent febrile attacks. Overall, at the end of the study, the prevalence of nephropathy was one third of that in a study conducted before Colchicine was used to treat familial Mediterranean fever. Among 960 patients who initially had no evidence of amyloidosis, proteinuria appeared in 4 who adhered to the prophylactic schedule and in 16 of 54 who admitted non-compliance. Life-table analysis showed that the cumulative rate of proteinuria was 1.7 percent (90 percent confidence limits, 0.0 and 11.3 percent) after 11 years in the compliant patients and 48.9 percent (18.8 and 79.0 percent) after 9 years in the noncompliant patients (P less than 0.0001). A total of 110 patients had overt nephropathy when they started to take Colchicine. Among 86 patients who had proteinuria but not the nephrotic syndrome, proteinuria resolved in 5 and stabilized in 68 (for more than eight years in 40). Renal function deteriorated in 13 of the patients with proteinuria and in all of the 24 patients with the nephrotic syndrome or uremia. We conclude that Colchicine prevented amyloidosis in our high-risk population and that it can prevent additional deterioration of renal function in patients with amyloidosis who have proteinuria but not the nephrotic syndrome.
Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain.[Pubmed:15014504]
Nature. 2004 Mar 11;428(6979):198-202.
Microtubules are cytoskeletal polymers of tubulin involved in many cellular functions. Their dynamic instability is controlled by numerous compounds and proteins, including Colchicine and stathmin family proteins. The way in which microtubule instability is regulated at the molecular level has remained elusive, mainly because of the lack of appropriate structural data. Here, we present the structure, at 3.5 A resolution, of tubulin in complex with Colchicine and with the stathmin-like domain (SLD) of RB3. It shows the interaction of RB3-SLD with two tubulin heterodimers in a curved complex capped by the SLD amino-terminal domain, which prevents the incorporation of the complexed tubulin into microtubules. A comparison with the structure of tubulin in protofilaments shows changes in the subunits of tubulin as it switches from its straight conformation to a curved one. These changes correlate with the loss of lateral contacts and provide a rationale for the rapid microtubule depolymerization characteristic of dynamic instability. Moreover, the tubulin-Colchicine complex sheds light on the mechanism of Colchicine's activity: we show that Colchicine binds at a location where it prevents curved tubulin from adopting a straight structure, which inhibits assembly.
Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils.[Pubmed:7543498]
J Clin Invest. 1995 Aug;96(2):994-1002.
Since Colchicine-sensitive microtubules regulate the expression and topography of surface glycoproteins on a variety of cells, we sought evidence that Colchicine interferes with neutrophil-endothelial interactions by altering the number and/or distribution of selectins on endothelial cells and neutrophils. Extremely low, prophylactic, concentrations of Colchicine (IC50 = 3 nM) eliminated the E-selectin-mediated increment in endothelial adhesiveness for neutrophils in response to IL-1 (P < 0.001) or TNF alpha (P < 0.001) by changing the distribution, but not the number, of E-selectin molecules on the surface of the endothelial cells. Colchicine inhibited stimulated endothelial adhesiveness via its effects on microtubules since vinblastine, an agent which perturbs microtubule function by other mechanisms, diminished adhesiveness whereas the photoinactivated Colchicine derivative gamma-lumiColchicine was inactive. Colchicine had no effect on cell viability. At higher, therapeutic, concentrations Colchicine (IC50 = 300 nM, P < 0.001) also diminished the expression of L-selectin on the surface of neutrophils (but not lymphocytes) without affecting expression of the beta 2-integrin CD11b/CD18. In confirmation, L-selectin expression was strikingly reduced (relative to CD11b/CD18 expression) on neutrophils from two individuals who had ingested therapeutic doses of Colchicine. These results suggest that Colchicine may exert its prophylactic effects on cytokine-provoked inflammation by diminishing the qualitative expression of E-selectin on endothelium, and its therapeutic effects by diminishing the quantitative expression of L-selectin on neutrophils.
Identification of cysteine 354 of beta-tubulin as part of the binding site for the A ring of colchicine.[Pubmed:8647876]
J Biol Chem. 1996 May 24;271(21):12639-45.
The Colchicine analog 3-chloroacetyl-3-demthylthio-Colchicine (3CTC) is a competitive inhibitor of Colchicine binding to tubulin, binds to tubulin at 37 degrees C, but not at 0 degree C, and covalently reacts with beta-tubulin at 37 degree C, but not at 0 degree C, in a reaction inhibited by Colchicine site drugs. The approximate intramolecular distance between the oxygen at position C-3 in 3CTC and the chlorine atom of the 3-chloroacetyl group is 3 A. using decylagarose chromatography, we purified beta-tubulin that had reacted with 3-(chloromethyl-[14C] Carbonyl)-3- demethylthioColchicine ([14C]3CTC). This beta-tubulin that had reacted with 3-(chloromethyl-[14C]carbonyl)- 3-demethythioColchicine ([14C]3CTC). This beta-tubulin was digested with formic acid, cyanogen bromide, endoproteinase Glu-C, or endoproteinase Lys-C, and the radio-labeled peptide(s) were isolated. The sequences of these peptides indicated that as much as 90% of the covalent reaction between the [14C]3CTC and beta-tubulin occurred at cysteine 354. This finding indicates that the C-3 oxygen atom of colchicinoids is within 3 A of the sulfur atom of the Cys-354 residue, suggests that the Colchicine A ring lies between Cys-354 and Cys-239, based on the known 9 A distance between these residues, and may indicate that the tropolone C ring lies between the peptide region containing Cys-239 and the amino-terminal beta-tubulin sequence, based on the labeling pattern observed following direct photoactivation of tubulin-bound Colchicine.
Analysis of the colchicine-binding site of beta-tubulin.[Pubmed:1544399]
FEBS Lett. 1992 Feb 10;297(3):205-8.
Comparison of the beta-tubulin sequences with the equilibrium Colchicine Ka and the Ki for inhibition by podophyllotoxin suggests that residue beta:316 is directly involved in binding the common trimethoxyphenyl-(or A-) ring. By contrast, the analysis indicates that the local hydrophobicity affects the rate of one of the two conformational changes associated with Colchicine binding but does not determine the affinity of the Colchicine-binding site.
Interactions of colchicine with tubulin.[Pubmed:1792241]
Pharmacol Ther. 1991;51(3):377-401.
Colchicine exerts its biological effects through binding to the soluble tubulin heterodimer, the major component of the microtubule. The Colchicine-binding abilities of tubulins from a variety of sources are summarized, and the mechanism of Colchicine binding to brain tubulin is explored in depth. The relationship between colchicinoid structure and tubulin binding activity provides insight into the structural features of Colchicine responsible for high affinity binding to tubulin and is reviewed for analogs in the Colchicine series. The thermodynamic and kinetic aspects of the association are described and evaluated in terms of the binding mechanism. Colchicine binding to tubulin results in unusual alterations in the low energy electronic spectra of Colchicine. The spectroscopic features of Colchicine bound to tubulin are discussed in terms of the nature of the Colchicine-tubulin complex. Attempts to locate the high affinity Colchicine binding site on tubulin are presented.


