NBD-556HIV-1 entry inhibitor,CD4 mimetic,block gp120-CD4 interaction CAS# 333353-44-9 |
- BMS-378806
Catalog No.:BCC4505
CAS No.:357263-13-9
- BMS-663068
Catalog No.:BCC1428
CAS No.:864953-29-7
- BMS-663068 Tris
Catalog No.:BCC1429
CAS No.:864953-39-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure
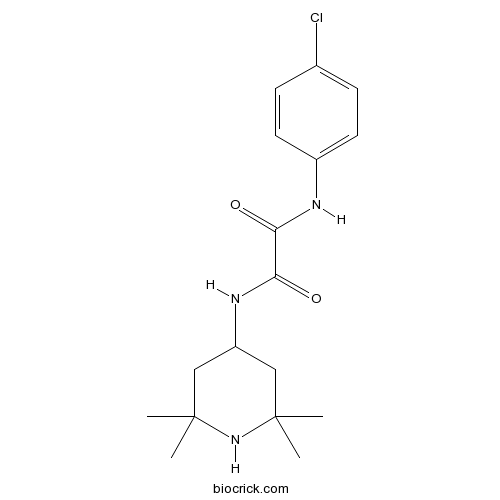
3D structure
| Cas No. | 333353-44-9 | SDF | Download SDF |
| PubChem ID | 1570601 | Appearance | Powder |
| Formula | C17H24ClN3O2 | M.Wt | 337.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (98.66 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | N'-(4-chlorophenyl)-N-(2,2,6,6-tetramethylpiperidin-4-yl)oxamide | ||
| SMILES | CC1(CC(CC(N1)(C)C)NC(=O)C(=O)NC2=CC=C(C=C2)Cl)C | ||
| Standard InChIKey | ZKXLQCIOURANAD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H24ClN3O2/c1-16(2)9-13(10-17(3,4)21-16)20-15(23)14(22)19-12-7-5-11(18)6-8-12/h5-8,13,21H,9-10H2,1-4H3,(H,19,22)(H,20,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CD4 mimetic (IC50 values are in low micromolar range, dependent on cell type and HIV strain). Recognizes and induces structural changes in HIV-1 envelope protein gp120, analogous to CD4 binding. Inhibits HIV-1 cell entry in CXCR4 and CCR5 expressing cell lines, by blocking virus-cell and cell-cell fusion. |

NBD-556 Dilution Calculator

NBD-556 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.96 mL | 14.7999 mL | 29.5998 mL | 59.1996 mL | 73.9995 mL |
| 5 mM | 0.592 mL | 2.96 mL | 5.92 mL | 11.8399 mL | 14.7999 mL |
| 10 mM | 0.296 mL | 1.48 mL | 2.96 mL | 5.92 mL | 7.4 mL |
| 50 mM | 0.0592 mL | 0.296 mL | 0.592 mL | 1.184 mL | 1.48 mL |
| 100 mM | 0.0296 mL | 0.148 mL | 0.296 mL | 0.592 mL | 0.74 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: NBD-556 inhibited cell–cell fusion between H9/HIV-1IIIB and MT-2 (IC50 ~3 μM)
The entry of HIV-1 into host cells is mediated by the binding of the surface subunit gp120 to the host cell receptor CD4. NBD-556 is a novel class of human immunodeficiency virus type 1 (HIV-1) entry inhibitor that block the gp120–CD4 interaction with drug-like properties.
In vitro: A systematic study showed that NBD-556 and NBD-557 target viral entry by inhibiting the binding of HIV-1 envelope glycoprotein gp120 to the cellular receptor CD4 but did not inhibit reverse transcriptase, protease, or integrase, demonstrating that they do not target the later stages of the HIV-1 life cycle to inhibit HIV-1 infection. NBD-556 and NBD-557 were also active against HIV-1 laboratory-adapted strains including an AZT-resistant strain and HIV-1 primary isolates, showing that these compounds can potentially be further modified to become potent HIV-1 entry inhibitors [1].
In vivo: No animal in-vivo data available currently.
Clinical trial: No clinical data are available.
Reference:
[1] Zhao, Q. , L. Ma, S. Jiang, H. Lu, S. Liu, Y. He, N. Strick, N. Neamati, and A. K. Debnath. 2005. Identification of N-phenyl-N′-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4. Virology 339:213-225.
- NBD-557
Catalog No.:BCC1791
CAS No.:333352-59-3
- Boc-Phe(4-NO2)-OH
Catalog No.:BCC3275
CAS No.:33305-77-0
- Dipsacoside B
Catalog No.:BCN5940
CAS No.:33289-85-9
- Gummiferin
Catalog No.:BCN8381
CAS No.:33286-30-5
- Diltiazem HCl
Catalog No.:BCC4901
CAS No.:33286-22-5
- 5,6-Dihydroyangonin
Catalog No.:BCN3566
CAS No.:3328-60-7
- 5-Aminofluorescein
Catalog No.:BCC8733
CAS No.:3326-34-9
- ML SA1
Catalog No.:BCC6276
CAS No.:332382-54-4
- Strychnistenolide
Catalog No.:BCN8039
CAS No.:332372-09-5
- 1,7-Bis(4-hydroxyphenyl)hepta-4,6-dien-3-one
Catalog No.:BCN7092
CAS No.:332371-82-1
- Rutaevin
Catalog No.:BCN6993
CAS No.:33237-37-5
- DZ2002
Catalog No.:BCC5544
CAS No.:33231-14-0
- Trianthenol
Catalog No.:BCN7802
CAS No.:333361-85-6
- Gliquidone
Catalog No.:BCC5003
CAS No.:33342-05-1
- Theaflavine-3,3'-digallate
Catalog No.:BCN5420
CAS No.:33377-72-9
- 9-O-Methyl-4-hydroxyboeravinone B
Catalog No.:BCN4063
CAS No.:333798-10-0
- Z-Asp(OtBu)-OSu
Catalog No.:BCC2787
CAS No.:3338-32-7
- 8-Deoxygartanin
Catalog No.:BCN5255
CAS No.:33390-41-9
- Gartanin
Catalog No.:BCN5256
CAS No.:33390-42-0
- LUF 5834
Catalog No.:BCC6237
CAS No.:333962-91-7
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Spermidine trihydrochloride
Catalog No.:BCC6865
CAS No.:334-50-9
- 3-Hydroxy-3-acetonyloxindole
Catalog No.:BCN4069
CAS No.:33417-17-3
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
Structure-Based Design of a Small Molecule CD4-Antagonist with Broad Spectrum Anti-HIV-1 Activity.[Pubmed:26301736]
J Med Chem. 2015 Sep 10;58(17):6909-6927.
Earlier we reported the discovery and design of NBD-556 and their analogs which demonstrated their potential as HIV-1 entry inhibitors. However, progress in developing these inhibitors has been stymied by their CD4-agonist properties, an unfavorable trait for use as drug. Here, we demonstrate the successful conversion of a full CD4-agonist (NBD-556) through a partial CD4-agonist (NBD-09027), to a full CD4-antagonist (NBD-11021) by structure-based modification of the critical oxalamide midregion, previously thought to be intolerant of modification. NBD-11021 showed unprecedented neutralization breath for this class of inhibitors, with pan-neutralization against a panel of 56 Env-pseudotyped HIV-1 representing diverse subtypes of clinical isolates (IC50 as low as 270 nM). The cocrystal structure of NBD-11021 complexed to a monomeric HIV-1 gp120 core revealed its detail binding characteristics. The study is expected to provide a framework for further development of NBD series as HIV-1 entry inhibitors for clinical application against AIDS.
CD4-induced activation in a soluble HIV-1 Env trimer.[Pubmed:24931470]
Structure. 2014 Jul 8;22(7):974-84.
The HIV envelope glycoprotein (Env) trimer undergoes receptor-induced conformational changes that drive fusion of the viral and cellular membranes. Env conformational changes have been observed using low-resolution electron microscopy, but only large-scale rearrangements have been visible. Here, we use hydrogen-deuterium exchange and oxidative labeling to gain a more precise understanding of the unliganded and CD4-bound forms of soluble Env trimers (SOSIP.664), including their glycan composition. CD4 activation induces the reorganization of bridging sheet elements, V1/V2 and V3, much of the gp120 inner domain, and the gp41 fusion subunit. Two CD4 binding site-targeted inhibitors have substantially different effects: NBD-556 partially mimics CD4-induced destabilization of the V1/V2 and V3 crown, whereas BMS-806 only affects regions around the gp120/gp41 interface. The structural information presented here increases our knowledge of CD4- and small molecule-induced conformational changes in Env and the allosteric pathways that lead to membrane fusion.
Discovery of small molecular inhibitors targeting HIV-1 gp120-CD4 interaction drived from BMS-378806.[Pubmed:25203778]
Eur J Med Chem. 2014 Oct 30;86:481-90.
The HIV-1 entry into host cells is a complex, multi-factors involved, and multi-step process. Especially, the attachment of HIV-1 envelope glycoprotein gp120 to the host cell receptor CD4 is the first key step during entry process, representing a promising antiviral therapeutic target. Among the HIV-1 attachment inhibitors blocking the interaction between gp120 and CD4 cells, BMS-378806 and NBD-556 are two representative small molecular chemical entities. Particularly, BMS-378806 and its derivatives are newly identified class of orally bioavailable HIV-1 inhibitors that interfere gp120-CD4 interaction. In this review, we focused on describing the structure-activity relationships (SARs), structural modifications, in vitro or even in vivo pharmacodynamics and pharmacokinetics of BMS-378806 and its analogues as HIV-1 gp120 attachment inhibitors. In addition, the brief SARs, structural modifications of NBD-556 and its derivatives targeting the "Phe-43 cavity" as CD4 mimics were also described.
Design, synthesis and evaluation of small molecule CD4-mimics as entry inhibitors possessing broad spectrum anti-HIV-1 activity.[Pubmed:27707628]
Bioorg Med Chem. 2016 Nov 15;24(22):5988-6003.
Since our first discovery of a CD4-mimic, NBD-556, which targets the Phe43 cavity of HIV-1 gp120, we and other groups made considerable progress in designing new CD4-mimics with viral entry-antagonist property. In our continued effort to make further progress we have synthesized twenty five new analogs based on our earlier reported viral entry antagonist, NBD-11021. These compounds were tested first in HIV-1 Env-pseudovirus based single-cycle infection assay as well as in a multi-cycle infection assay. Four of these new compounds showed much improved antiviral potency as well as cytotoxicity. We selected two of the best compounds 45A (NBD-14009) and 46A (NBD-14010) to test against a panel of 51 Env-pseudotyped HIV-1 representing diverse subtypes of clinical isolates. These compounds showed noticeable breadth of antiviral potency with IC50 of as low as 150nM. These compounds also inhibited cell-to-cell fusion and cell-to-cell HIV-1 transmission. The study is expected to pave the way of designing more potent and selective HIV-1 entry inhibitors targeted to the Phe43 cavity of HIV-1 gp120.
Binding mode characterization of NBD series CD4-mimetic HIV-1 entry inhibitors by X-ray structure and resistance study.[Pubmed:25001301]
Antimicrob Agents Chemother. 2014 Sep;58(9):5478-91.
We previously identified two small-molecule CD4 mimetics--NBD-556 and NBD-557--and synthesized a series of NBD compounds that resulted in improved neutralization activity in a single-cycle HIV-1 infectivity assay. For the current investigation, we selected several of the most active compounds and assessed their antiviral activity on a panel of 53 reference HIV-1 Env pseudoviruses representing diverse clades of clinical isolates. The selected compounds inhibited tested clades with low-micromolar potencies. Mechanism studies indicated that they act as CD4 agonists, a potentially unfavorable therapeutic trait, in that they can bind to the gp120 envelope glycoprotein and initiate a similar physiological response as CD4. However, one of the compounds, NBD-09027, exhibited reduced agonist properties, in both functional and biophysical studies. To understand the binding mode of these inhibitors, we first generated HIV-1-resistant mutants, assessed their behavior with NBD compounds, and determined the X-ray structures of two inhibitors, NBD-09027 and NBD-10007, in complex with the HIV-1 gp120 core at approximately 2-A resolution. Both studies confirmed that the NBD compounds bind similarly to NBD-556 and NBD-557 by inserting their hydrophobic groups into the Phe43 cavity of gp120. The basic nitrogen of the piperidine ring is located in close proximity to D368 of gp120 but it does not form any H-bond or salt bridge, a likely explanation for their nonoptimal antagonist properties. The results reveal the structural and biological character of the NBD series of CD4 mimetics and identify ways to reduce their agonist properties and convert them to antagonists.
Peptides from second extracellular loop of C-C chemokine receptor type 5 (CCR5) inhibit diverse strains of HIV-1.[Pubmed:22403408]
J Biol Chem. 2012 Apr 27;287(18):15076-86.
To initiate HIV entry, the HIV envelope protein gp120 must engage its primary receptor CD4 and a coreceptor CCR5 or CXCR4. In the absence of a high resolution structure of a gp120-coreceptor complex, biochemical studies of CCR5 have revealed the importance of its N terminus and second extracellular loop (ECL2) in binding gp120 and mediating viral entry. Using a panel of synthetic CCR5 ECL2-derived peptides, we show that the C-terminal portion of ECL2 (2C, comprising amino acids Cys-178 to Lys-191) inhibit HIV-1 entry of both CCR5- and CXCR4-using isolates at low micromolar concentrations. In functional viral assays, these peptides inhibited HIV-1 entry in a CD4-independent manner. Neutralization assays designed to measure the effects of CCR5 ECL2 peptides when combined with either with the small molecule CD4 mimetic NBD-556, soluble CD4, or the CCR5 N terminus showed additive inhibition for each, indicating that ECL2 binds gp120 at a site distinct from that of N terminus and acts independently of CD4. Using saturation transfer difference NMR, we determined the region of CCR5 ECL2 used for binding gp120, showed that it can bind to gp120 from both R5 and X4 isolates, and demonstrated that the peptide interacts with a CD4-gp120 complex in a similar manner as to gp120 alone. As the CCR5 N terminus-gp120 interactions are dependent on CD4 activation, our data suggest that gp120 has separate binding sites for the CCR5 N terminus and ECL2, the ECL2 binding site is present prior to CD4 engagement, and it is conserved across CCR5- and CXCR4-using strains. These peptides may serve as a starting point for the design of inhibitors with broad spectrum anti-HIV activity.
Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops.[Pubmed:22451932]
Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5663-8.
The HIV-1 envelope (Env) spike (gp120(3)/gp41(3)) undergoes considerable structural rearrangements to mediate virus entry into cells and to evade the host immune response. Engagement of CD4, the primary human receptor, fixes a particular conformation and primes Env for entry. The CD4-bound state, however, is prone to spontaneous inactivation and susceptible to antibody neutralization. How does unliganded HIV-1 maintain CD4-binding capacity and regulate transitions to the CD4-bound state? To define this mechanistically, we determined crystal structures of unliganded core gp120 from HIV-1 clades B, C, and E. Notably, all of these unliganded HIV-1 structures resembled the CD4-bound state. Conformational fixation with ligand selection and thermodynamic analysis of full-length and core gp120 interactions revealed that the tendency of HIV-1 gp120 to adopt the CD4-bound conformation was restrained by the V1/V2- and V3-variable loops. In parallel, we determined the structure of core gp120 in complex with the small molecule, NBD-556, which specifically recognizes the CD4-bound conformation of gp120. Neutralization by NBD-556 indicated that Env spikes on primary isolates rarely assume the CD4-bound conformation spontaneously, although they could do so when quaternary restraints were loosened. Together, the results suggest that the CD4-bound conformation represents a "ground state" for the gp120 core, with variable loop and quaternary interactions restraining unliganded gp120 from "snapping" into this conformation. A mechanism of control involving deformations in unliganded structure from a functionally critical state (e.g., the CD4-bound state) provides advantages in terms of HIV-1 Env structural diversity and resistance to antibodies and inhibitors, while maintaining elements essential for entry.
Identification of N-phenyl-N'-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4.[Pubmed:15996703]
Virology. 2005 Sep 1;339(2):213-25.
We have identified two N-phenyl-N'-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamide analogs as a novel class of human immunodeficiency virus type 1 (HIV-1) entry inhibitors that block the gp120-CD4 interaction, using database screening techniques. The lead compounds, NBD-556 and NBD-557, are small molecule organic compounds with drug-like properties. These compounds showed potent cell fusion and virus-cell fusion inhibitory activity at low micromolar levels. A systematic study showed that these compounds target viral entry by inhibiting the binding of HIV-1 envelope glycoprotein gp120 to the cellular receptor CD4 but did not inhibit reverse transcriptase, integrase, or protease, indicating that they do not target the later stages of the HIV-1 life cycle to inhibit HIV-1 infection. These compounds were equally potent inhibitors of both X4 and R5 viruses tested in CXCR4 and CCR5 expressing cell lines, respectively, indicating that their anti-HIV-1 activity is not dependent on the coreceptor tropism of the virus. A surface plasmon resonance study, which measures binding affinity, clearly demonstrated that these compounds bind to unliganded HIV-1 gp120 but not to the cellular receptor CD4. NBD-556 and NBD-557 were active against HIV-1 laboratory-adapted strains including an AZT-resistant strain and HIV-1 primary isolates, indicating that these compounds can potentially be further modified to become potent HIV-1 entry inhibitors.


