MRS 2500 tetraammonium saltExtremely potent and selective P2Y1 antagonist CAS# 630103-23-0 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 630103-23-0 | SDF | Download SDF |
| PubChem ID | 90488745 | Appearance | Powder |
| Formula | C13H30IN9O8P2 | M.Wt | 629.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
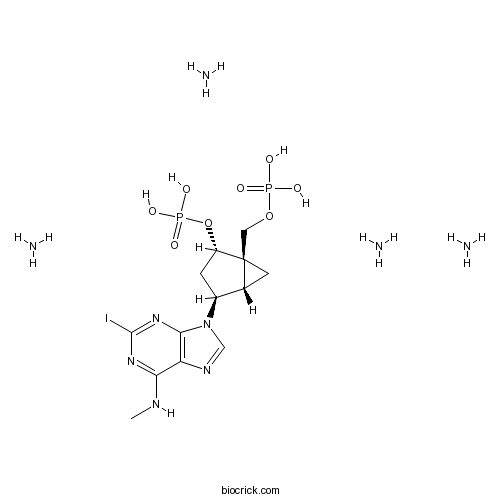
| Chemical Name | azane;[(1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)purin-9-yl]-2-phosphonooxy-1-bicyclo[3.1.0]hexanyl]methyl dihydrogen phosphate | ||
| SMILES | CNC1=NC(=NC2=C1N=CN2C3CC(C4(C3C4)COP(=O)(O)O)OP(=O)(O)O)I.N.N.N.N | ||
| Standard InChIKey | FVTFHHDVLNQSME-AVAGOIHISA-N | ||
| Standard InChI | InChI=1S/C13H18IN5O8P2.4H3N/c1-15-10-9-11(18-12(14)17-10)19(5-16-9)7-2-8(27-29(23,24)25)13(3-6(7)13)4-26-28(20,21)22;;;;/h5-8H,2-4H2,1H3,(H,15,17,18)(H2,20,21,22)(H2,23,24,25);4*1H3/t6-,7+,8+,13+;;;;/m1..../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent and selective antagonist of the platelet P2Y1 receptor (Ki = 0.78 nM). Inhibits ADP-induced aggregation of human platelets with an IC50 value of 0.95 nM. Inhibits the upregulation of NTPDase1 by ATPγS. Prevents thrombus formation in vivo. |

MRS 2500 tetraammonium salt Dilution Calculator

MRS 2500 tetraammonium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5891 mL | 7.9455 mL | 15.8909 mL | 31.7818 mL | 39.7273 mL |
| 5 mM | 0.3178 mL | 1.5891 mL | 3.1782 mL | 6.3564 mL | 7.9455 mL |
| 10 mM | 0.1589 mL | 0.7945 mL | 1.5891 mL | 3.1782 mL | 3.9727 mL |
| 50 mM | 0.0318 mL | 0.1589 mL | 0.3178 mL | 0.6356 mL | 0.7945 mL |
| 100 mM | 0.0159 mL | 0.0795 mL | 0.1589 mL | 0.3178 mL | 0.3973 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neoprzewaquinone A
Catalog No.:BCN4169
CAS No.:630057-39-5
- Corynoxeine
Catalog No.:BCN5002
CAS No.:630-94-4
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
- Ouabain
Catalog No.:BCC5069
CAS No.:630-60-4
- Nonacosane
Catalog No.:BCC9102
CAS No.:630-03-5
- Phenoxybenzamine HCl
Catalog No.:BCC4334
CAS No.:63-92-3
- L-Phenylalanine
Catalog No.:BCN3818
CAS No.:63-91-2
- Sulfanilamide
Catalog No.:BCC4858
CAS No.:63-74-1
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Primaquine Diphosphate
Catalog No.:BCC4706
CAS No.:63-45-6
- Androstenedione
Catalog No.:BCC8296
CAS No.:63-05-8
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- PD 168077 maleate
Catalog No.:BCC6919
CAS No.:630117-19-0
- AST 487
Catalog No.:BCC1373
CAS No.:630124-46-8
- Androstenone hydrazone
Catalog No.:BCC8830
CAS No.:63015-10-1
- Crenulatin
Catalog No.:BCN7791
CAS No.:63026-02-8
- Hexacosyl (E)-ferulate
Catalog No.:BCN4170
CAS No.:63034-29-7
- Senkyunolide
Catalog No.:BCN8154
CAS No.:63038-10-8
- H-Tle-OMe.HCl
Catalog No.:BCC2658
CAS No.:63038-27-7
- Estradiol-3-benzoate-17-butyrate
Catalog No.:BCC8963
CAS No.:63042-18-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- H-Val-OMe.HCl
Catalog No.:BCC3142
CAS No.:6306-52-1
- Boc-Thr-OSu
Catalog No.:BCC3450
CAS No.:63076-44-8
- (±)-threo-3-Methylglutamic acid
Catalog No.:BCC6804
CAS No.:63088-04-0
Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells.[Pubmed:17626796]
J Pharmacol Exp Ther. 2007 Oct;323(1):157-64.
Stimulation of receptors for either ATP or adenosine leads to physiologic changes in retinal pigment epithelial (RPE) cells that may influence their relationship with the adjacent photoreceptors. The ectoenzyme nucleoside-triphosphate diphosphohydrolase-1 (NTPDase1) catalyzes the dual dephosphorylation of ATP and ADP to AMP. Although NTPDase1 can consequently control the balance between ATP and adenosine, it is unclear how its expression and activity are regulated. Classic negative feedback theory predicts an increase in enzyme activity in response to enhanced exposure to substrate. This study asked whether exposure to ATP increases NTPDase1 activity in RPE cells. Although levels of NTPDase1 mRNA and protein in cultured human ARPE-19 cells were generally low under control conditions, exposure to slowly hydrolyzable ATPgammaS led to a time-dependent increase in NTPDase1 mRNA that was accompanied by a rise in levels of the functional 78-kDa protein. Neither NTPDase2 nor NTPDase3 mRNA message was elevated by ATPgammaS. The ATPase activity of cells increased in parallel, indicating the up-regulation of NTPDase1 was functionally relevant. The up-regulation of NTPDase1 protein was partially blocked by P2Y1 receptor inhibitors MRS2179 (N6-methyl-2'-deoxyadenosine-3',5'-bisphosphate) and MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2'-deoxyadenosine 3',5'-bisphosphate] and increased by P2Y1 receptor agonist MRS2365 [(N)-methanocarba-2MeSADP]. In conclusion, prolonged exposure to extracellular ATPgammaS increased NTPDase1 message and protein levels and increased ecto-ATPase activity. This up-regulation reflects a feedback circuit, mediated at least in part by the P2Y1 receptor, to regulate levels of extracellular purines in subretinal space. NTPDase1 levels may thus serve as an index for increased extracellular ATP levels under certain pathologic conditions, although other mechanisms could also contribute.
MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice.[Pubmed:16236815]
J Pharmacol Exp Ther. 2006 Feb;316(2):556-63.
The platelet P2Y(1) ADP receptor is an attractive target for new antiplatelet drugs. However, because of the lack of strong and stable antagonists, only a few studies have suggested that pharmacological inhibition of the P2Y(1) receptor could efficiently inhibit experimental thrombosis in vivo. Our aim was to determine whether the newly described potent and selective P2Y(1) receptor antagonist MRS2500 [2-iodo-N(6)-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate] could inhibit platelet function ex vivo and experimental thrombosis in mice in vivo. MRS2500 was injected intravenously into mice, and its effect on ex vivo platelet aggregation and in several models of thrombosis in vivo was determined. MRS2500 displayed high potency and stable and selective P2Y(1) receptor inhibition ex vivo. Although MRS2500 injection resulted in only moderate prolongation of the bleeding time, it provided strong protection in systemic thromboembolism induced by infusion of a mixture of collagen and adrenaline. MRS2500 also potently inhibited localized arterial thrombosis in a model of laser-induced vessel wall injury with two degrees of severity. Moreover, combination of MRS2500 with clopidogrel, the irreversible inhibitor of the platelet P2Y(12) receptor for ADP, led to increased antithrombotic efficacy compared with each alone. These results add further evidence for a role of the P2Y(1) receptor in thrombosis and validate the concept that targeting the P2Y(1) receptor could be a relevant alternative or complement to current antiplatelet strategies.
Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor.[Pubmed:15476670]
Biochem Pharmacol. 2004 Nov 15;68(10):1995-2002.
Activation of the P2Y(1) nucleotide receptor in platelets by ADP causes changes in shape and aggregation, mediated by activation of phospholipase C (PLC). Recently, MRS2500(2-iodo-N(6)-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate) was introduced as a highly potent and selective antagonist for this receptor. We have studied the actions of MRS2500 in human platelets and compared these effects with the effects of two acyclic nucleotide analogues, a bisphosphate MRS2298 and a bisphosphonate derivative MRS2496, which act as P2Y(1) receptor antagonists, although less potently than MRS2500. Improved synthetic methods for MRS2500 and MRS2496 were devised. The bisphosphonate is predicted to be more stable in general in biological systems than phosphate antagonists due to the non-hydrolyzable CP bond. MRS2500 inhibited the ADP-induced aggregation of human platelets with an IC(50) value of 0.95 nM. MRS2298 and MRS2496 also both inhibited the ADP-induced aggregation of human platelets with IC(50) values of 62.8 nM and 1.5 microM, respectively. A similar order of potency was observed for the three antagonists in binding to the recombinant human P2Y(1) receptor and in inhibition of ADP-induced shape change and ADP-induced rise in intracellular Ca(2+). No substantial antagonism of the pathway linked to the inhibition of cyclic AMP was observed for the nucleotide derivatives, indicating no interaction of these three P2Y(1) receptor antagonists with the proaggregatory P2Y(12) receptor, which is also activated by ADP. Thus, all three of the bisphosphate derivatives are highly selective antagonists of the platelet P2Y(1) receptor, and MRS2500 is the most potent such antagonist yet reported.
2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists.[Pubmed:14584948]
J Med Chem. 2003 Nov 6;46(23):4974-87.
Preference for the northern (N) ring conformation of the ribose moiety of adenine nucleotide 3',5'-bisphosphate antagonists of P2Y(1) receptors was established by using a ring-constrained methanocarba (a bicyclo[3.1.0]hexane) ring as a ribose substitute (Nandanan et al. J. Med. Chem. 2000, 43, 829-842). We have now combined the ring-constrained (N)-methanocarba modification with other functionalities at the 2-position of the adenine moiety. A new synthetic route to this series of bisphosphate derivatives was introduced, consisting of phosphorylation of the pseudoribose moiety prior to coupling with the adenine base. The activity of the newly synthesized analogues was determined by measuring antagonism of 2-methylthio-ADP-stimulated phospholipase C (PLC) activity in 1321N1 human astrocytoma cells expressing the recombinant human P2Y(1) receptor and by using the radiolabeled antagonist [(3)H]2-chloro-N(6)-methyl-(N)-methanocarba-2'-deoxyadenosine 3',5'-bisphosphate 5 in a newly developed binding assay in Sf9 cell membranes. Within the series of 2-halo analogues, the most potent molecule at the hP2Y(1) receptor was an (N)-methanocarba N(6)-methyl-2-iodo analogue 12, which displayed a K(i) value in competition for binding of [(3)H]5 of 0.79 nM and a K(B) value of 1.74 nM for inhibition of PLC. Thus, 12 is the most potent antagonist selective for the P2Y(1) receptor yet reported. The 2-iodo group was substituted with trimethyltin, thus providing a parallel synthetic route for the introduction of an iodo group in this high-affinity antagonist. The (N)-methanocarba-2-methylthio, 2-methylseleno, 2-hexyl, 2-(1-hexenyl), and 2-(1-hexynyl) analogues bound less well, exhibiting micromolar affinity at P2Y(1) receptors. An enzymatic method of synthesis of the 3',5'-bisphosphate from the corresponding 3'-monophosphate, suitable for the preparation of a radiophosphorylated analogue, was explored.


