mGlu2 agonistAnti-depressants,novel potent agent CAS# 1311385-32-6 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- MPEP Hydrochloride
Catalog No.:BCC1777
CAS No.:219911-35-0
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1311385-32-6 | SDF | Download SDF |
| PubChem ID | 66577008 | Appearance | Powder |
| Formula | C13H22N6O6S | M.Wt | 390.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (128.07 mM) DMSO : < 1 mg/mL (insoluble or slightly soluble) *"≥" means soluble, but saturation unknown. | ||
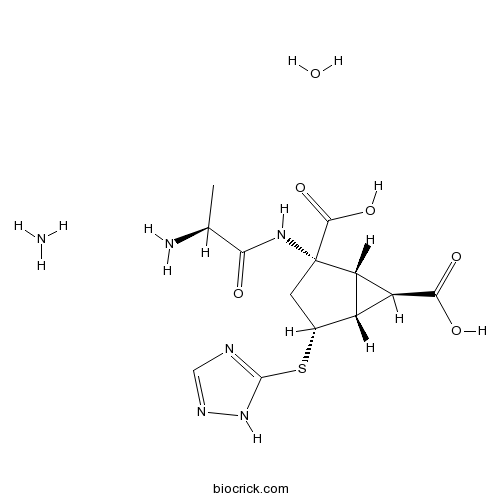
| Chemical Name | (1R,2S,4R,5R,6R)-2-[[(2S)-2-aminopropanoyl]amino]-4-(1H-1,2,4-triazol-5-ylsulfanyl)bicyclo[3.1.0]hexane-2,6-dicarboxylic acid;azane;hydrate | ||
| SMILES | CC(C(=O)NC1(CC(C2C1C2C(=O)O)SC3=NC=NN3)C(=O)O)N.N.O | ||
| Standard InChIKey | FICWTZOQUXUYOK-AHKKVLALSA-N | ||
| Standard InChI | InChI=1S/C13H17N5O5S.H3N.H2O/c1-4(14)9(19)17-13(11(22)23)2-5(24-12-15-3-16-18-12)6-7(8(6)13)10(20)21;;/h3-8H,2,14H2,1H3,(H,17,19)(H,20,21)(H,22,23)(H,15,16,18);1H3;1H2/t4-,5+,6-,7-,8-,13-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY2979165 is a mGlu2 agonist, which is a novel potent agent that is used as anti-depressants.
IC50 Value:
Target: mGluR References: | |||||

mGlu2 agonist Dilution Calculator

mGlu2 agonist Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5613 mL | 12.8067 mL | 25.6134 mL | 51.2269 mL | 64.0336 mL |
| 5 mM | 0.5123 mL | 2.5613 mL | 5.1227 mL | 10.2454 mL | 12.8067 mL |
| 10 mM | 0.2561 mL | 1.2807 mL | 2.5613 mL | 5.1227 mL | 6.4034 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5123 mL | 1.0245 mL | 1.2807 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2561 mL | 0.5123 mL | 0.6403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2979165 is a novel potent agent that is used as Anti-depressants. LY2979165 is under clinical study. A Safety Study of LY2979165 is performing in Healthy Subjects. LY2979165 is useful for Anti-depressants.
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- Miltipolone
Catalog No.:BCN3222
CAS No.:131086-61-8
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- GNF179 Metabolite
Catalog No.:BCC5176
CAS No.:1310455-86-7
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- 8-Epiloganic acid
Catalog No.:BCC8956
CAS No.:82509-41-9
- Meptyldinocap
Catalog No.:BCC5468
CAS No.:131-72-6
- Oxybenzone
Catalog No.:BCC5445
CAS No.:131-57-7
- N-Acetylneuraminic acid
Catalog No.:BCN2204
CAS No.:131-48-6
- Sodium Demethylcantharidate
Catalog No.:BCN8394
CAS No.:13114-29-9
- NSC 624206
Catalog No.:BCC7988
CAS No.:13116-77-3
- Cornuside
Catalog No.:BCN5007
CAS No.:131189-57-6
- PF 5081090
Catalog No.:BCC6148
CAS No.:1312473-63-4
- Antibiotic PF 1018
Catalog No.:BCN2149
CAS No.:131256-42-3
- Scutebarbatine W
Catalog No.:BCN7011
CAS No.:1312716-25-8
- Scutebarbatine X
Catalog No.:BCN6997
CAS No.:1312716-26-9
- Scutebarbatine Y
Catalog No.:BCN6994
CAS No.:1312716-27-0
- Scutebarbatine Z
Catalog No.:BCN6991
CAS No.:1312716-28-1
- Ro 25-6981 Maleate
Catalog No.:BCC4159
CAS No.:1312991-76-6
- Ro 8-4304 hydrochloride
Catalog No.:BCC7655
CAS No.:1312991-77-7
- threo Ifenprodil hemitartrate
Catalog No.:BCC7508
CAS No.:1312991-83-5
Evaluation of single and multiple doses of a novel mGlu2 agonist, a potential antipsychotic therapy, in healthy subjects.[Pubmed:28156011]
Br J Clin Pharmacol. 2017 Aug;83(8):1654-1667.
AIMS: The safety, tolerability, pharmacokinetics (PK) and pharmacodynamics of single and multiple doses of a novel mGlu2 agonist were assessed in healthy males. METHODS: In two, Phase 1 investigator- and subject-blind, placebo-controlled studies, oral doses of prodrug LY2979165 were evaluated: single doses (20-150 mg, N = 30) and multiple once-daily (QD) doses (20-400 mg; N = 84), using a titration regimen. The plasma and urine PK of LY2979165 and active moiety, 2812223, were measured. Cerebrospinal fluid (CSF) was collected to determine PK and neurotransmitter levels. Safety parameters were assessed throughout. RESULTS: Nausea and vomiting were dose limiting following single doses; dose titration allowed higher doses to be tested over 14 days. The most common adverse events related to LY2979165 were dizziness, vomiting, nausea, somnolence and headache. The plasma PK of 2812223 were approximately linear with minimal accumulation with QD dosing. Conversion of LY2979165 to 2812223 was extensive, with minimal LY2979165 measurable in plasma. There was no effect of food on the PK of LY2979165 and 2812223. After 60 mg LY2979165 single-dose, 2812223 exposure in CSF was approximately 2-6% and plasma exposure and peak concentrations were approximately four-fold higher than the mGlu2 agonist in vitro EC50 value. No consistent effects were observed on CSF neurotransmitter levels. CONCLUSIONS: Oral doses of LY2979165 up to 60 mg as a single dose and up to 400 mg given as multiple QD doses, using a titration regimen, were well tolerated with linear PK. Overall, these data support further clinical evaluation of LY2979165.
Synthesis and Pharmacological Characterization of C4-(Thiotriazolyl)-substituted-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylates. Identification of (1R,2S,4R,5R,6R)-2-Amino-4-(1H-1,2,4-triazol-3-ylsulfanyl)bicyclo[3.1.0]hexane-2, 6-dicarboxylic Acid (LY2812223), a Highly Potent, Functionally Selective mGlu2 Receptor Agonist.[Pubmed:26313429]
J Med Chem. 2015 Sep 24;58(18):7526-48.
Identification of orthosteric mGlu(2/3) receptor agonists capable of discriminating between individual mGlu2 and mGlu3 subtypes has been highly challenging owing to the glutamate-site sequence homology between these proteins. Herein we detail the preparation and characterization of a series of molecules related to (1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylate 1 (LY354740) bearing C4-thiotriazole substituents. On the basis of second messenger responses in cells expressing other recombinant human mGlu2/3 subtypes, a number of high potency and efficacy mGlu2 receptor agonists exhibiting low potency mGlu3 partial agonist/antagonist activity were identified. From this, (1R,2S,4R,5R,6R)-2-amino-4-(1H-1,2,4-triazol-3-ylsulfanyl)bicyclo[3.1.0]hexane-2, 6-dicarboxylic acid 14a (LY2812223) was further characterized. Cocrystallization of 14a with the amino terminal domains of hmGlu2 and hmGlu3 combined with site-directed mutation studies has clarified the underlying molecular basis of this unique pharmacology. Evaluation of 14a in a rat model responsive to mGlu2 receptor activation coupled with a measure of central drug disposition provides evidence that this molecule engages and activates central mGlu2 receptors in vivo.
Synthesis and pharmacological characterization of C4-disubstituted analogs of 1S,2S,5R,6S-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylate: identification of a potent, selective metabotropic glutamate receptor agonist and determination of agonist-bound human mGlu2 and mGlu3 amino terminal domain structures.[Pubmed:25602126]
J Med Chem. 2015 Feb 26;58(4):1776-94.
As part of our ongoing research to identify novel agents acting at metabotropic glutamate 2 (mGlu2) and 3 (mGlu3) receptors, we have previously reported the identification of the C4alpha-methyl analog of mGlu2/3 receptor agonist 1 (LY354740). This molecule, 1S,2S,4R,5R,6S-2-amino-4-methylbicyclo[3.1.0]hexane-2,6-dicarboxylate 2 (LY541850), exhibited an unexpected mGlu2 agonist/mGlu3 antagonist pharmacological profile, whereas the C4beta-methyl diastereomer (3) possessed dual mGlu2/3 receptor agonist activity. We have now further explored this structure-activity relationship through the preparation of cyclic and acyclic C4-disubstituted analogs of 1, leading to the identification of C4-spirocyclopropane 5 (LY2934747), a novel, potent, and systemically bioavailable mGlu2/3 receptor agonist which exhibits both antipsychotic and analgesic properties in vivo. In addition, through the combined use of protein-ligand X-ray crystallography employing recombinant human mGlu2/3 receptor amino terminal domains, molecular modeling, and site-directed mutagenesis, a molecular basis for the observed pharmacological profile of compound 2 is proposed.
Dissociation of mGlu2/3 agonist effects on ketamine-induced regional and event-related oxygen signals.[Pubmed:25943169]
Psychopharmacology (Berl). 2015 Nov;232(21-22):4219-29.
RATIONALE: Validating preclinical biomarkers that predict treatment efficacy remains a critical imperative for neuropsychiatric drug discovery. With the establishment of novel in vivo imaging methods, it has become possible to think how such translational proof-of-concept studies may look. OBJECTIVES: The aim of this study was to use in vivo oxygen (O2) amperometry to simultaneously assess the regional and event/task-related O2 changes induced by ketamine challenge in rats, and to determine whether both of these signals are equivalently affected by the mGlu2/3 receptor agonist LY379268. METHODS: O2 signals were measured via carbon paste electrodes implanted in the anterior cingulate cortex (ACC) of rats trained to perform a simple reaction time task (SRT). SRT performance, event-related ACC O2 responses, and regional ACC O2 signal were recorded simultaneously in animals treated with ketamine (10 mg/kg) and/or LY379268 (3 mg/kg). RESULTS: A consistent relationship was observed between baseline SRT performance and related ACC O2 signals, suggesting that ACC engagement is likely to be a requirement for optimal task performance. Ketamine induced a robust and consistent slowing in reaction times that was reflected by a delayed event-related ACC O2 signal increase compared to vehicle controls. Ketamine also produced a regional and task-independent 60-min increase in ACC O2 levels which was effectively attenuated by LY379268. However, LY379238 failed to reverse alterations in event-related O2 signals and associated SRT task performance. CONCLUSIONS: These findings raise questions about the degree to which such reversals of regional ketamine O2 signals could potentially be claimed to predict drug treatment efficacy.


