Licorisoflavan ACAS# 129314-37-0 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 129314-37-0 | SDF | Download SDF |
| PubChem ID | 196831 | Appearance | Powder |
| Formula | C27H34O5 | M.Wt | 438.6 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
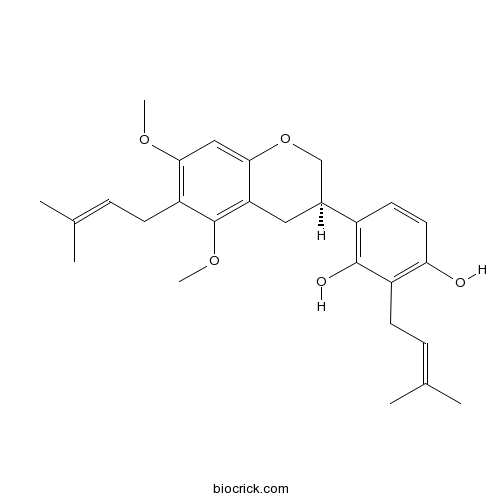
| Chemical Name | 4-[(3R)-5,7-dimethoxy-6-(3-methylbut-2-enyl)-3,4-dihydro-2H-chromen-3-yl]-2-(3-methylbut-2-enyl)benzene-1,3-diol | ||
| SMILES | CC(=CCC1=C(C=CC(=C1O)C2CC3=C(C(=C(C=C3OC2)OC)CC=C(C)C)OC)O)C | ||
| Standard InChIKey | GDAAEAXMNLVRCZ-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C27H34O5/c1-16(2)7-9-20-23(28)12-11-19(26(20)29)18-13-22-25(32-15-18)14-24(30-5)21(27(22)31-6)10-8-17(3)4/h7-8,11-12,14,18,28-29H,9-10,13,15H2,1-6H3/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Licorisoflavan A shows weak scavenging activity against superoxide anion radical. 2. Licorisoflavan A has bactericidal effects on S. mutans UA159 at the concentration of ≥4 g/ml, it can be useful in developing oral hygiene products, such as gargling solutions and dentifrices for preventing dental caries. 3. Licorisoflavan A and licoricidin have potential for the development of novel host-modulating strategies for the treatment of cytokine and/or MMP-mediated disorders such as periodontitis. |
| Targets | p65 | NF-kB | AP-1 | MMP(e.g.TIMP) | IL Receptor |

Licorisoflavan A Dilution Calculator

Licorisoflavan A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.28 mL | 11.3999 mL | 22.7998 mL | 45.5996 mL | 56.9995 mL |
| 5 mM | 0.456 mL | 2.28 mL | 4.56 mL | 9.1199 mL | 11.3999 mL |
| 10 mM | 0.228 mL | 1.14 mL | 2.28 mL | 4.56 mL | 5.7 mL |
| 50 mM | 0.0456 mL | 0.228 mL | 0.456 mL | 0.912 mL | 1.14 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.228 mL | 0.456 mL | 0.57 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoangustone A
Catalog No.:BCN6819
CAS No.:129280-34-8
- Semilicoisoflavone B
Catalog No.:BCN2931
CAS No.:129280-33-7
- PF-2545920
Catalog No.:BCC2279
CAS No.:1292799-56-4
- Boscialin
Catalog No.:BCN7323
CAS No.:129277-03-8
- Pierreione B
Catalog No.:BCN6855
CAS No.:1292766-21-2
- Perospirone hydrochloride
Catalog No.:BCC9118
CAS No.:129273-38-7
- Hoechst 33258 analog 6
Catalog No.:BCC1628
CAS No.:129244-66-2
- IKKε-IN-1
Catalog No.:BCC5514
CAS No.:1292310-49-6
- 15-Dihydroepioxylubimin
Catalog No.:BCN4800
CAS No.:129214-59-1
- (2S,3S)-(-)-Glucodistylin
Catalog No.:BCN6156
CAS No.:129212-92-6
- Iberiotoxin
Catalog No.:BCC6932
CAS No.:129203-60-7
- Biotin-HPDP
Catalog No.:BCC3583
CAS No.:129179-83-5
- Alendronate sodium
Catalog No.:BCC3719
CAS No.:129318-43-0
- O-Geranylconiferyl alcohol
Catalog No.:BCN4747
CAS No.:129350-09-0
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
- Fmoc-Phenylalaninol
Catalog No.:BCC2717
CAS No.:129397-83-7
- Boc-Cysteinol(pMeBzl)
Catalog No.:BCC3044
CAS No.:129397-85-9
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Fmoc-Asp-OtBu
Catalog No.:BCC3088
CAS No.:129460-09-9
- 3,4-Diacetoxycinnamamide
Catalog No.:BCN6157
CAS No.:129488-34-2
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Tolfenpyrad
Catalog No.:BCC8069
CAS No.:129558-76-5
In vitro antimicrobial activities of 1-methoxyficifolinol, licorisoflavan A, and 6,8-diprenylgenistein against Streptococcus mutans.[Pubmed:25531232]
Caries Res. 2015;49(1):78-89.
The objective of the study was to investigate the antimicrobial effects of purified single compounds from ethanol-extracted licorice root on Streptococcus mutans. The crude licorice root extract (CLE) was obtained from Glycyrrhiza uralensis, which was subjected to column chromatography to separate compounds. Purified compounds were identified by mass spectrometry and nuclear magnetic resonance. Antimicrobial activities of purified compounds from CLE were evaluated by determining the minimum inhibitory concentration and by performing time-kill kinetics. The inhibitory effects of the compounds on biofilm development were evaluated using crystal violet assay and confocal microscopy. Cell toxicity of substances to normal human gingival fibroblast (NHGF) cells was tested using a methyl thiazolyl tetrazolium assay. Chlorhexidine digluconate (CHX) was used in the control group. Three antimicrobial flavonoids, 1-methoxyficifolinol, Licorisoflavan A, and 6,8-diprenylgenistein, were isolated from the CLE. We found that the three flavonoids and CHX had bactericidal effects on S. mutans UA159 at the concentration of >/=4 and >/=1 microg/ml, respectively. The purified compounds completely inhibited biofilm development of S. mutans UA159 at concentrations over 4 mug/ml, which was equivalent to 2 mug/ml of CHX. Confocal analysis showed that biofilms were sparsely scattered in the presence of over 4 mug/ml of the purified compounds. However, the three compounds purified from CLE showed less cytotoxic effects on NHGF cells than CHX at these biofilm-inhibitory concentrations. Our results suggest that purified flavonoids from CLE can be useful in developing oral hygiene products, such as gargling solutions and dentifrices for preventing dental caries.
Antinephritis and radical scavenging activity of prenylflavonoids.[Pubmed:14630182]
Fitoterapia. 2003 Dec;74(7-8):720-4.
Antinephritis activity of 5 prenylflavonoids similar to glabridin (1-5), isolated from Morus alba, Artocarpus communis, Glycyrrhiza uralensis and G. inflata, was evaluated in mice with glomerular disease (Masugi-nephritis). Oral administrations of artonin E (2) or licochalcone A (4) for 10 days (30 mg kg(-1) day(-1)) reduced the amount of urinary protein excretion compared to nephritic mice. ESR spectroscopy demonstrated that morusin (1) and Licorisoflavan A (5) increased the radical intensity of sodium ascorbate by about two times. Morusin, licoricidin (3), licochalcone A and Licorisoflavan A showed weak scavenging activity against superoxide anion radical.
Modulation of matrix metalloproteinase and cytokine production by licorice isolates licoricidin and licorisoflavan A: potential therapeutic approach for periodontitis.[Pubmed:20722535]
J Periodontol. 2011 Jan;82(1):122-8.
BACKGROUND: Inflammatory cytokines and matrix metalloproteinases (MMPs) produced by resident and inflammatory cells in response to periodontopathogens play a major role in the tissue destruction observed in periodontitis, which is a disease that affects tooth-supporting structures. In the present study, we investigate the effects of licorice-derived licoricidin (LC) and Licorisoflavan A (LIA) on the secretion of various cytokines and MMPs by human monocyte-derived macrophages stimulated with Aggregatibacter actinomycetemcomitans (previously Actinobacillus actinomycetemcomitans) lipopolysaccharide (LPS). METHODS: Macrophages were treated with non-toxic concentrations of LC or LIA before being stimulated with A. actinomycetemcomitans LPS. The secretion of cytokines and MMPs and the activation of nuclear factor-kappa B (NF-kappaB) p65 and activator protein (AP)-1 were assessed by enzyme-linked immunosorbent assays. RESULTS: LC and LIA inhibited the secretion of interleukin (IL)-6 and chemokine (C-C motif) ligand 5 in a concentration-dependent manner but did not affect the secretion of IL-8 by LPS-stimulated macrophages. LC and LIA also inhibited the secretion of MMP-7, -8, and -9 by macrophages. The suppression of cytokine and MMP secretion by LC and LIA was associated with the reduced activation of NF-kappaB p65 but not that of AP-1. CONCLUSION: The present study suggests that LC and LIA have potential for the development of novel host-modulating strategies for the treatment of cytokine and/or MMP-mediated disorders such as periodontitis.


