VerteporfinPhotosensitizer used in photodynamic therapy CAS# 129497-78-5 |
- Dorzolamide HCl
Catalog No.:BCC2311
CAS No.:130693-82-2
Quality Control & MSDS
Number of papers citing our products

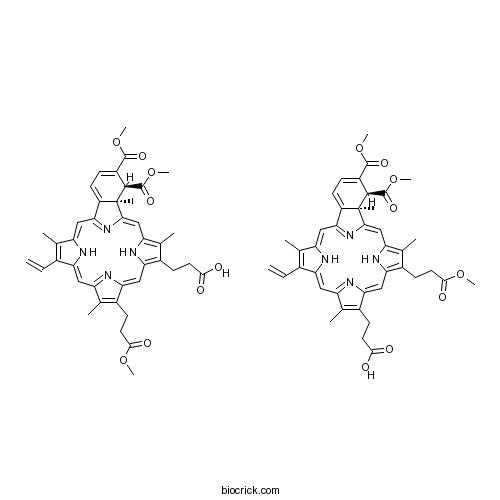
Chemical structure
3D structure
| Cas No. | 129497-78-5 | SDF | Download SDF |
| PubChem ID | 11980904 | Appearance | Powder |
| Formula | C41H42N4O8 | M.Wt | 718.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Visudyne | ||
| Solubility | DMF : 25 mg/mL (34.78 mM; Need ultrasonic) DMSO : 8 mg/mL (11.13 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | CC1=C(C2=CC3=NC(=CC4=C(C(=C(N4)C=C5C6(C(C(=CC=C6C(=N5)C=C1N2)C(=O)OC)C(=O)OC)C)C)CCC(=O)OC)C(=C3C)CCC(=O)O)C=C.CC1=C(C2=CC3=NC(=CC4=C(C(=C(N4)C=C5C6(C(C(=CC=C6C(=N5)C=C1N2)C(=O)OC)C(=O)OC)C)C)CCC(=O)O)C(=C3C)CCC(=O)OC)C=C | ||
| Standard InChIKey | NJLRKAMQPVVOIU-IDLGWYNRSA-N | ||
| Standard InChI | InChI=1S/2C41H42N4O8/c1-9-23-20(2)29-17-34-27-13-10-26(39(49)52-7)38(40(50)53-8)41(27,5)35(45-34)19-30-22(4)24(11-14-36(46)47)32(44-30)18-33-25(12-15-37(48)51-6)21(3)28(43-33)16-31(23)42-29;1-9-23-20(2)29-17-34-27-13-10-26(39(49)52-7)38(40(50)53-8)41(27,5)35(45-34)19-30-22(4)25(12-15-37(48)51-6)33(44-30)18-32-24(11-14-36(46)47)21(3)28(43-32)16-31(23)42-29/h2*9-10,13,16-19,38,42,44H,1,11-12,14-15H2,2-8H3,(H,46,47)/t2*38-,41+/m00/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | YAP inhibitor; disrupts YAP-TEAD interactions. Enhances trypsin cleavage of YAP (EC50 = 100 nM). Inhibits growth and proliferation of retinoblastoma cells. Also suppresses cancer stem cell properties in vitro. Suppresses YAP-induced liver overgrowth in mice. |

Verteporfin Dilution Calculator

Verteporfin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3912 mL | 6.9561 mL | 13.9123 mL | 27.8245 mL | 34.7807 mL |
| 5 mM | 0.2782 mL | 1.3912 mL | 2.7825 mL | 5.5649 mL | 6.9561 mL |
| 10 mM | 0.1391 mL | 0.6956 mL | 1.3912 mL | 2.7825 mL | 3.4781 mL |
| 50 mM | 0.0278 mL | 0.1391 mL | 0.2782 mL | 0.5565 mL | 0.6956 mL |
| 100 mM | 0.0139 mL | 0.0696 mL | 0.1391 mL | 0.2782 mL | 0.3478 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Verteporfin, derived from porphyrin, is a potent photosensitizing agent, which has all the features theoretically necessary for effective photodynamic therapy (PDT) in ocular neovascularization [1].
Experimentas showed that the mechanism of action of verteporfin therapy is intravascular damage leading to the formation of thrombus and selective vascular occlusion. Photodynamic therapy with verteporfin triggers cellular events consistent with a number of the changes described for cells rendered by different chemotherapeutic agents.
More than 90% of HL-60 cells treated with verteporfin at 100 ng/mL showed a hypodiploid level of DNA, when approximately 25% of irradiated cells treated with verteporfin at 50 ng/mL exhibited DNA fragmentation. For cells treated with verteporfin at 25 ng/mL, there was an 85% or greater loss in viability as determined by MTT assays performed 24 hours after irradiation. A 5–6 hours of the plasma half-life of verteporfin was shown in a pharmacokinetic studies in humans. At 6 mg/m2, which is the clinically relevant dose being used in neovascular AMD, no skin photosensitivity was detected at 24 hours, whereas at 18 mg/m2, baseline was reached by 5 days [1, 2].
Verteporfin was also found to inhibit autophagosome formation that does not require exposure to light. Verteporfin could target and modify p62 directly, a protein of scaffold and adaptor that binds both polyubiquitinated proteins destined for degradation and LC3 on autophagosomal membranes. Co-immunoprecipitation experiments demonstrated that crosslinked p62 oligomers retain their ability to bind to LC3 but show defective binding to polyubiquitinated proteins. Interestingly, small amounts of crosslinked p62 oligomers were detected in cells which were untreated, and other groups which were noted the accumulation of p62 forms with reduced SDS-PAGE mobility in cellular and animal models of oxidative stress and aging [3].
References:
[1].Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Survey Of Ophthalmology, 2000, 45(3): 195-214.
[2]. Granville DJ, Carthy CM, Jiang H, et al. Nuclear factor-kappa B activation by the photochemotherapeutic agent verteporfin. Blood, 2000, 95(1): 256-262.
[3]. Donohue E, Balgi AD, Komatsu M, et al. Induction of covalently crosslinked p62 oligomers with reduced binding to polyubiquitinated proteins by the autophagy inhibitor verteporfin. PLoS ONE, 2014, 9(12): e114964.
- 3,4-Diacetoxycinnamamide
Catalog No.:BCN6157
CAS No.:129488-34-2
- Fmoc-Asp-OtBu
Catalog No.:BCC3088
CAS No.:129460-09-9
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Boc-Cysteinol(pMeBzl)
Catalog No.:BCC3044
CAS No.:129397-85-9
- Fmoc-Phenylalaninol
Catalog No.:BCC2717
CAS No.:129397-83-7
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- O-Geranylconiferyl alcohol
Catalog No.:BCN4747
CAS No.:129350-09-0
- Alendronate sodium
Catalog No.:BCC3719
CAS No.:129318-43-0
- Licorisoflavan A
Catalog No.:BCN6662
CAS No.:129314-37-0
- Isoangustone A
Catalog No.:BCN6819
CAS No.:129280-34-8
- Tolfenpyrad
Catalog No.:BCC8069
CAS No.:129558-76-5
- Goniopypyrone
Catalog No.:BCN3957
CAS No.:129578-07-0
- Satraplatin
Catalog No.:BCC5356
CAS No.:129580-63-8
- Nevirapine
Catalog No.:BCC3820
CAS No.:129618-40-2
- GR 82334
Catalog No.:BCC5802
CAS No.:129623-01-4
- 3-Hydroxybisabola-1,10-dien-9-one
Catalog No.:BCN7325
CAS No.:129673-86-5
- 3,4-Dihydroxybisabola-1,10-diene
Catalog No.:BCN7326
CAS No.:129673-87-6
- ZD 7114 hydrochloride
Catalog No.:BCC6852
CAS No.:129689-28-7
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
Primary photodynamic therapy with verteporfin for small pigmented posterior pole choroidal melanoma.[Pubmed:28338667]
Eye (Lond). 2017 Apr;31(4):519-528.
PurposeThe purpose of the study was to investigate the outcomes of primary photodynamic therapy (PDT) for small pigmented posterior pole choroidal melanoma.Patients and methodsProspective interventional consecutive case series of 15 patients with small pigmented posterior pole choroidal melanoma, who were treated with three sessions of PDT and followed-up thereafter. Risk factors for failure were assessed and outcome measures at presentation were compared to those at last follow-up visit.ResultsTumor control was achieved in 12 (80%) patients in a median follow-up time of 15 months (mean 14, range 8-18). Three patients failed treatment, diagnosed in a median time of 5 months (mean 4, range 3-6), after first PDT. In all failed cases, lesions were 100% pigmented; de novo melanoma rather than transformed nevi and showed a radial growth pattern rather than increased thickness. All failed cases were subsequently successfully treated with radiotherapy. In this cohort, subretinal fluid (SRF) was significantly reduced (P<0.001), vision did not deteriorate (P=0.11) and even improved in patients with subfoveal SRF at presentation (P=0.018), tumor height significantly decreased (P=0.037) and no complications were recorded.ConclusionPrimary PDT was found to be a safe and efficient treatment modality for small pigmented posterior pole choroidal melanoma, achieving short-term tumor control in 80% of patients. PDT offers patients the opportunity to preserve vision by avoiding the retinopathy associated with conventional radiation treatments for choroidal melanoma. However, the long-term local control of these tumors remains uncertain.
Combination verteporfin photodynamic therapy ranibizumab-dexamethasone in choroidal neovascularization due to age-related macular degeneration: results of a phase II randomized trial.[Pubmed:28182161]
Clin Ophthalmol. 2017 Jan 24;11:223-231.
PURPOSE: To assess whether combination therapy (CT) reduces retreatments when compared to ranibizumab monotherapy (RM), while safely maintaining similar vision outcomes. METHODS: In this 24-month trial, patients with age-related macular degeneration (AMD) were randomized to 1) quarter-fluence or 2) half-fluence triple therapy (Verteporfin photodynamic therapy [vPDT] + ranibizumab + dexamethasone), 3) half-fluence double therapy (vPDT + ranibizumab), or 4) RM. The primary outcomes were number of retreatment visits and change from baseline in visual acuity (VA) at 12 months. RESULTS: One hundred sixty-two subjects enrolled. There were 4.0 (P=0.02), 3.2 (P<0.001), 4.1 (P=0.03), and 5.7 retreatment visits through month 12, and 5.9 (P=0.03), 4.3 (P<0.001), 5.9 (P=0.02) and 8.7 through month 24, in groups 1, 2, 3, and 4, respectively (P-value comparing with RM). Month 12 VA score change from baseline (95% confidence interval) was +3.6 (-0.9 to +8.1), +6.8 (+2.4 to +11.1), +5.0 (+0.6 to +9.3), and +6.5 (+1.7 to +11.4), respectively. CONCLUSION: CT resulted in significantly fewer retreatment visits than a RM regimen at months 12 and 24. VA results appeared similar although wide confidence intervals preclude conclusions regarding vision outcomes.
Molecular Features of the YAP Inhibitor Verteporfin: Synthesis of Hexasubstituted Dipyrrins as Potential Inhibitors of YAP/TAZ, the Downstream Effectors of the Hippo Pathway.[Pubmed:28334506]
ChemMedChem. 2017 Jun 21;12(12):954-961.
Porphyrin derivatives, in particular Verteporfin (VP), a photosensitizer initially designed for cancer therapy, have been identified as inhibitors of the YAP-TEAD interaction and transcriptional activity. Herein we report the efficient convergent synthesis of the dipyrrin half of protoporphyrin IX dimethyl ester (PPIX-DME), in which the sensitive vinyl group was created at the final stage by a dehydroiodination reaction. Two other dipyrrin derivatives were synthesized, including dipyrrin 19 [(Z)-2-((3,5-dimethyl-4-vinyl-2H-pyrrol-2-ylidene)methyl)-3,5-dimethyl-4-vinyl-1H -pyrrole], containing two vinyl groups. We found that VP and dipyrrin 19 showed significant inhibitory effects on TEAD transcriptional activity in MDA-MB-231 human breast cancer cells, whereas other compounds did not show significant changes. In addition, we observed a marked decrease in both YAP and TAZ levels following VP treatment, whereas dipyrrin 19 treatment primarily decreased the levels of YAP and receptor kinase AXL, a downstream target of YAP. Together, our data suggest that, due to their chemical structures, porphyrin- and dipyrrin-related derivatives can directly target YAP and/or TAZ proteins and inhibit TEAD transcriptional activity.
Verteporfin induces apoptosis and eliminates cancer stem-like cells in uveal melanoma in the absence of light activation.[Pubmed:28042502]
Am J Cancer Res. 2016 Dec 1;6(12):2816-2830. eCollection 2016.
Uveal melanoma (UM) is the most common primary ocular malignancy in adults. Currently, no beneficial systemic therapy is available; therefore, there is an urgent need for effective targeted therapeutic drugs. As Verteporfin has shown anti-neoplastic activity in several types of cancers, here we hypothesized and investigated the efficacy of Verteporfin against UM cells without light activation. MTS assay, flow cytometry analysis of apoptosis, Western blotting of relevant proteins, transwell migration and invasion assay, melanosphere culture, and measurement of ALDH(+) populations, were used to evaluate the effects of Verteporfin on UM cells. We found that Verteporfin disrupted the interaction between YAP and TEAD4 in UM cells and decreased the expression of YAP targeted downstream genes. Verteporfin treatment decreased the cytoplasmic and nuclear levels of YAP and induced lysosome-dependent degradation of YAP protein. Verteporfin exhibited distinct inhibitory effect on the proliferation of four lines of UM cells (e.g., 92.1, Mel 270, Omm 1 and Omm 2.3), and induced apoptosis through the intrinsic pathway. Additionally, Verteporfin suppressed migration and invasion of UM cells, impaired the traits of cancer stem-like cells (e.g., melanosphere formation capacity, and ALDH(+) cell population). This study demonstrated the anti-neoplastic activity of Verteporfin against UM cells in vitro, providing a rationale for evaluating this agent in clinical investigation.
The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation.[Pubmed:24837142]
Exp Eye Res. 2014 Jul;124:67-73.
Verteporfin (VP), a benzoporphyrin derivative, is clinically used in photodynamic therapy for neovascular macular degeneration. Recent studies indicate that VP may inhibit growth of hepatoma cells without photoactivation through inhibition of YAP-TEAD complex. In this study, we examined the effects of VP without light activation on human retinoblastoma cell lines. Verteporfin but not vehicle control inhibited the growth, proliferation and viability of human retinoblastoma cell lines (Y79 and WERI) in a dose-dependent manner and was associated with downregulation of YAP-TEAD associated downstream proto-oncogenes such as c-myc, Axl, and surviving. In addition VP affected signals involved in cell migration and angiogenesis such as CTGF, cyr61, and VEGF-A but was not associated with significant effect on the mTOR/autophagy pathway. Of interest the pluripotency marker Oct4 were downregulated by Verteporfin treatment. Our results indicate that the clinically used photosensitizer VP is a potent inhibitor of cell growth in retinoblastoma cells, disrupting YAP-TEAD signaling and pluripotential marker OCT4. This study highlights for the first time the role of the YAP-TEAD pathway in Retinoblastoma and suggests that VP may be a useful adjuvant therapeutic tool in treating Rb patients.
Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties.[Pubmed:24906622]
Cancer Res. 2014 Aug 1;74(15):4170-82.
Cancer stem cells (CSC) are purported to initiate and maintain tumor growth. Deregulation of normal stem cell signaling may lead to the generation of CSCs; however, the molecular determinants of this process remain poorly understood. Here we show that the transcriptional coactivator YAP1 is a major determinant of CSC properties in nontransformed cells and in esophageal cancer cells by direct upregulation of SOX9. YAP1 regulates the transcription of SOX9 through a conserved TEAD binding site in the SOX9 promoter. Expression of exogenous YAP1 in vitro or inhibition of its upstream negative regulators in vivo results in elevated SOX9 expression accompanied by the acquisition of CSC properties. Conversely, shRNA-mediated knockdown of YAP1 or SOX9 in transformed cells attenuates CSC phenotypes in vitro and tumorigenicity in vivo. The small-molecule inhibitor of YAP1, Verteporfin, significantly blocks CSC properties in cells with high YAP1 and a high proportion of ALDH1(+). Our findings identify YAP1-driven SOX9 expression as a critical event in the acquisition of CSC properties, suggesting that YAP1 inhibition may offer an effective means of therapeutically targeting the CSC population.
Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP.[Pubmed:22677547]
Genes Dev. 2012 Jun 15;26(12):1300-5.
The Drosophila TEAD ortholog Scalloped is required for Yki-mediated overgrowth but is largely dispensable for normal tissue growth, suggesting that its mammalian counterpart may be exploited for selective inhibition of oncogenic growth driven by YAP hyperactivation. Here we test this hypothesis genetically and pharmacologically. We show that a dominant-negative TEAD molecule does not perturb normal liver growth but potently suppresses hepatomegaly/tumorigenesis resulting from YAP overexpression or Neurofibromin 2 (NF2)/Merlin inactivation. We further identify Verteporfin as a small molecule that inhibits TEAD-YAP association and YAP-induced liver overgrowth. These findings provide proof of principle that inhibiting TEAD-YAP interactions is a pharmacologically viable strategy against the YAP oncoprotein.


