Hoechst 33258 analog 6Blue fluorescent dyes CAS# 129244-66-2 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 129244-66-2 | SDF | Download SDF |
| PubChem ID | 71576675 | Appearance | Powder |
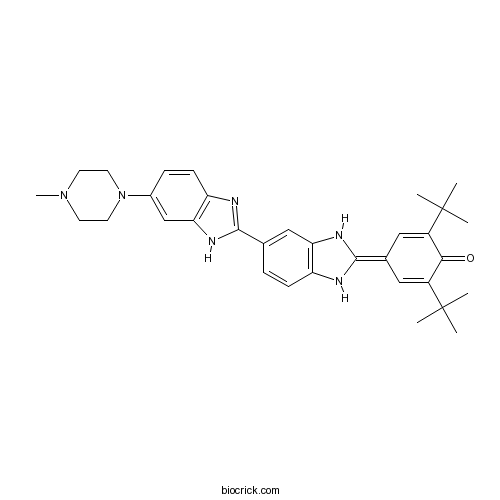
| Formula | C33H40N6O | M.Wt | 536.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO or water Protect from light | ||
| Chemical Name | 2,6-ditert-butyl-4-[5-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1,3-dihydrobenzimidazol-2-ylidene]cyclohexa-2,5-dien-1-one | ||
| SMILES | CC(C)(C)C1=CC(=C2NC3=C(N2)C=C(C=C3)C4=NC5=C(N4)C=C(C=C5)N6CCN(CC6)C)C=C(C1=O)C(C)(C)C | ||
| Standard InChIKey | LQFJIAYWATWTRS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C33H40N6O/c1-32(2,3)23-16-21(17-24(29(23)40)33(4,5)6)31-35-25-10-8-20(18-27(25)36-31)30-34-26-11-9-22(19-28(26)37-30)39-14-12-38(7)13-15-39/h8-11,16-19,35-36H,12-15H2,1-7H3,(H,34,37) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hoechst 33258 analog 6 Dilution Calculator

Hoechst 33258 analog 6 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8632 mL | 9.316 mL | 18.632 mL | 37.2641 mL | 46.5801 mL |
| 5 mM | 0.3726 mL | 1.8632 mL | 3.7264 mL | 7.4528 mL | 9.316 mL |
| 10 mM | 0.1863 mL | 0.9316 mL | 1.8632 mL | 3.7264 mL | 4.658 mL |
| 50 mM | 0.0373 mL | 0.1863 mL | 0.3726 mL | 0.7453 mL | 0.9316 mL |
| 100 mM | 0.0186 mL | 0.0932 mL | 0.1863 mL | 0.3726 mL | 0.4658 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C. The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably. Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. in vitro: N/A in vivo: N/A Clinical trial: N/A
- IKKε-IN-1
Catalog No.:BCC5514
CAS No.:1292310-49-6
- 15-Dihydroepioxylubimin
Catalog No.:BCN4800
CAS No.:129214-59-1
- (2S,3S)-(-)-Glucodistylin
Catalog No.:BCN6156
CAS No.:129212-92-6
- Iberiotoxin
Catalog No.:BCC6932
CAS No.:129203-60-7
- Biotin-HPDP
Catalog No.:BCC3583
CAS No.:129179-83-5
- Gancaonin M
Catalog No.:BCN4757
CAS No.:129145-51-3
- 2-(2,2-Dimethyl-1,3-dioxolan-4-yl)propane-1,2-diol
Catalog No.:BCC8475
CAS No.:129141-48-6
- CGRP 8-37 (rat)
Catalog No.:BCC5717
CAS No.:129121-73-9
- ENMD-2076 L-(+)-Tartaric acid
Catalog No.:BCC2185
CAS No.:1291074-87-7
- Evodosin A
Catalog No.:BCN7322
CAS No.:1291053-38-7
- Rivastigmine Tartrate
Catalog No.:BCC3851
CAS No.:129101-54-8
- Buclizine HCl
Catalog No.:BCC4516
CAS No.:129-74-8
- Perospirone hydrochloride
Catalog No.:BCC9118
CAS No.:129273-38-7
- Pierreione B
Catalog No.:BCN6855
CAS No.:1292766-21-2
- Boscialin
Catalog No.:BCN7323
CAS No.:129277-03-8
- PF-2545920
Catalog No.:BCC2279
CAS No.:1292799-56-4
- Semilicoisoflavone B
Catalog No.:BCN2931
CAS No.:129280-33-7
- Isoangustone A
Catalog No.:BCN6819
CAS No.:129280-34-8
- Licorisoflavan A
Catalog No.:BCN6662
CAS No.:129314-37-0
- Alendronate sodium
Catalog No.:BCC3719
CAS No.:129318-43-0
- O-Geranylconiferyl alcohol
Catalog No.:BCN4747
CAS No.:129350-09-0
- 11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one
Catalog No.:BCC8430
CAS No.:129385-59-7
- Physcion 8-O-rutinoside
Catalog No.:BCN7324
CAS No.:129393-21-1
- Euphorbia factor L9
Catalog No.:BCN3786
CAS No.:129393-28-8
Hydroquinone analog 4-[(Tetrahydro-2H-pyran-2yl) oxy] phenol induces C26 colon cancer cell apoptosis and inhibits tumor growth in vivo.[Pubmed:25651526]
Mol Med Rep. 2015 Jun;11(6):4671-7.
The 4[(Tetrahydro2Hpyran2yl) oxy] phenol (XGd) hydroquinone analog, is found in Vaccinium vitisidaea L. Although it is known for its antioxidant properties and high level of safety, its antitumor activity remains to be elucidated. In the present study, the anticancer effect of XGd was determined in vitro and in vivo. The cytotoxicity of XGd against C26 murine colon carcinoma cells was found to occur in a time and concentrationdependent manner, whereas little effect was observed in the two normal cell lines (HK2 and L02) investigated. Oral administration of XGd (100 mg/kg) had effects on the tumor growth of tumorbearing mice. Furthermore, marked apoptosis was observed using Hoechst 33258 staining and flow cytometric analysis with annexin V/propidium iodide double staining. XGd also downregulated the expression of Bcell lymphoma 2 (Bcl2), increased the expression levels of Bcl2associated X protein and activated caspase9, caspase3 and poly(adenosine diphosphateribose) polymerase. The present study demonstrated for the first time, to the best of our knowledge, that XGd inhibited cancer cell growth via the induction of apoptosis and was also able to inhibit tumor growth in vivo. These results demonstrated that XGd may be used as a potential natural agent for cancer therapy with low toxicity.
Dynamic proliferation assessment in flow cytometry.[Pubmed:20853345]
Curr Protoc Cell Biol. 2010 Sep;Chapter 8:Unit 8.6.1-23.
Dynamic proliferation assessment via flow cytometry is legitimately supposed to be the most powerful tool for recording cell cycle kinetics in-vitro. The preeminent feature is a single cell-based multi-informative analysis by temporal high-resolution. Flow cytometric approaches are based on labeling of proliferating cells via thymidine substitution by a base analog (e.g., 5-bromo-2'-deoxyuridine, BrdU) that is added to cell cultures either for a short period of time (pulse labeling) or continuously until cell harvesting. This unit describes the alternative use of the thymidine analog 5-ethynyl-2'-deoxyuridine (EdU) in place of BrdU for three different applications: (1) dynamic proliferation assessment by EdU pulse cell labeling; (2) the same approach as (1) but in combination with live/dead cell discrimination; and (3) dynamic cell cycle analysis based on continuous cell labeling with EdU and Hoechst fluorochrome quenching. In contrast to the detection of BrdU incorporation, EdU-positive cells can be identified by taking advantage of click chemistry, which facilitates a simplified and fast cell preparation. Further analysis options but also limitations of the utilization of EdU are discussed.
Induction of apoptosis in human renal cell carcinoma cells by vitamin E succinate in caspase-independent manner.[Pubmed:18692875]
Urology. 2009 Jan;73(1):193-9.
OBJECTIVES: Renal cell carcinoma (RCC) is one of the most drug-resistant malignancies, and an effective therapy is lacking for metastatic RCC. Vitamin E (VE) has been intensively studied as a chemopreventive agent for various cancer types. Preclinical investigations have suggested that VE succinate (VES) is the most effective analog of VE in cancer cells; however, no study of VES in RCC has been done. We investigated the anticancer activity of VES against RCC. METHODS: Cytotoxicity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell morphologic changes and cell viability were evaluated using phase-contrast microscopy and the trypan blue dye-exclusion test, respectively. Caspase activity was measured with a quantitative colorimetric assay. RESULTS: VES exerted dose- and time-dependent cytotoxicities against ACHN, a human RCC cell line, but VE and VE acetate did not. The cytotoxic effect was also observed in 2 other RCC cell lines, Caki-1 and Caki-2, and in primary RCC cells derived from 8 patients. Hoechst 33258 staining and DNA ladder analysis demonstrated that VES induced apoptosis in RCC cells. However, VES did not affect activation of caspase-3, -6, -8, or -9. Furthermore, inhibitors specific to caspase-8, -9, -6, and -3 did not block VES cytotoxicity and neither did the general caspase inhibitor VAD. CONCLUSIONS: VES might induce apoptosis and cytotoxicity against RCC cells in a caspase-independent manner and has potential in vivo applications in the treatment of drug-and/or immunotherapy-resistant RCC.
Early redistribution of plasma membrane phosphatidylserine during apoptosis of adult rat ventricular myocytes in vitro.[Pubmed:10424235]
Basic Res Cardiol. 1999 Jun;94(3):171-9.
In many cell types, DNA fragmentation is a late event of apoptosis which may be lacking. This contrasts with the early translocation of phosphatidylserine (PS) from the internal to the external leaflet of the cell membrane. We examined whether an early PS translocation also occurs during apoptosis induced in adult rat ventricular myocytes grown in the presence of 10% fetal calf serum (FCS), by the protein kinase inhibitor staurosporine. Apoptosis was assessed by the observation of: (i) typical alterations in cell morphology; (ii) nuclear alterations visualized using the permeant intercalating agent Hoechst 33258; (iii) DNA fragmentation detected by the TUNEL method. PS translocation was detected using annexin V binding. Data are expressed as means +/- SEM. Prolonged exposure of myocytes to 10 microM staurosporine from day 3 to day 7 of culture resulted in cell shrinkage, typical nuclear alterations, membrane protrusions and fragmentation of the sarcomeric apparatus in the vast majority of myocytes. At this time, 52.4 +/- 5.7% of staurosporine-treated myocytes were TUNEL positive (vs 6.1 +/- 2.0% in control cultures (CC), p < 0.001) and 69.7 +/- 1.7% were annexin V positive (vs 21.1 +/- 1.0% in CC, p < 0.001). Importantly, PS translocation was detected as early as 35 minutes following staurosporine addition, the percentage of annexin V positive myocytes reaching 10 times the control value (19.2 +/- 2.7 vs 1.8 +/- 0.8%, p < 0.001) after 3 hours. A 18-hour staurosporine exposure of freshly isolated myocytes resulted, at the end of exposure, in 24.3 +/- 1.7% annexin V positive myocytes (vs 9.6 +/- 0.5% in CC, p < 0.05), whereas a marked increase in the percentage of TUNEL positive myocytes was observed only from day 5. Finally, myocyte exposure to the membrane-permeant ceramide analog, C2-ceramide (50 microM), resulted in 63.2 +/- 3.5% annexin V positive myocytes 4 hours later (vs 17.8 +/- 4.4% in CC, p < 0.001), whereas a significant increase in the percentage of TUNEL positive myocytes was detected only the next day (43.7 +/- 3.4 vs 9.9 +/- 1.3%, p < 0.001). Taken together, these results strongly suggest that the loss of PS asymmetry is an early event of cardiac myocyte apoptosis which precedes DNA fragmentation.
Analysis of sister-chromatid exchanges.[Pubmed:18428316]
Curr Protoc Hum Genet. 2001 May;Chapter 8:Unit 8.6.
Two requirements for the cytogenetic analysis of sister-chromatid exchanges (SCEs) in somatic cells are (1) a population of actively proliferating cells that will provide an adequate number of metaphases and (2) sister chromatids that in some way are differentially labeled or stained in the metaphases. SCEs can be recognized as abrupt discontinuities in the staining patterns of the two chromatids of a metaphase chromosome at what appear to be identical sites, with reciprocal switching from one chromatid to its sister. This protocol uses phytohemagglutinin (PHA)-stimulated cultures of blood lymphocytes as a source of proliferating cells. The cells are incubated with the thymidine analog BrdU. Slides prepared from fixed cells with BrdU-substituted chromosomes are treated with Hoechst 33258, exposed to light and heat, and then Giemsa-stained to produce differentially stained chromosomes. The chromatids with bifilar substitution exhibit a lighter purple stain than their unifilarly substituted sister chromatids.


