EGLUHighly selective group II mGlu antagonist CAS# 170984-72-2 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 170984-72-2 | SDF | Download SDF |
| PubChem ID | 5311079 | Appearance | Powder |
| Formula | C7H13NO4 | M.Wt | 175.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
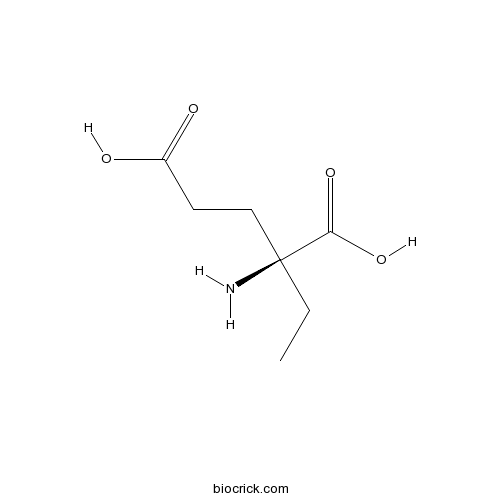
| Chemical Name | (2S)-2-amino-2-ethylpentanedioic acid | ||
| SMILES | CCC(CCC(=O)O)(C(=O)O)N | ||
| Standard InChIKey | QFYBYZLHPIALCZ-ZETCQYMHSA-N | ||
| Standard InChI | InChI=1S/C7H13NO4/c1-2-7(8,6(11)12)4-3-5(9)10/h2-4,8H2,1H3,(H,9,10)(H,11,12)/t7-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective antagonist of presynaptically-mediated (1S,3S)-ACPD-induced depression of motoneuron excitation in neonatal rat spinal cord; presumed group II mGlu receptor antagonist. Also available as part of the Group II mGlu Receptor. |

EGLU Dilution Calculator

EGLU Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.7084 mL | 28.5421 mL | 57.0841 mL | 114.1683 mL | 142.7104 mL |
| 5 mM | 1.1417 mL | 5.7084 mL | 11.4168 mL | 22.8337 mL | 28.5421 mL |
| 10 mM | 0.5708 mL | 2.8542 mL | 5.7084 mL | 11.4168 mL | 14.271 mL |
| 50 mM | 0.1142 mL | 0.5708 mL | 1.1417 mL | 2.2834 mL | 2.8542 mL |
| 100 mM | 0.0571 mL | 0.2854 mL | 0.5708 mL | 1.1417 mL | 1.4271 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tetrindole mesylate
Catalog No.:BCC6763
CAS No.:170964-68-8
- Dihydroactinidiolide
Catalog No.:BCN6890
CAS No.:17092-92-1
- Donitriptan hydrochloride
Catalog No.:BCC7742
CAS No.:170911-68-9
- Aburatubolactam A
Catalog No.:BCN1821
CAS No.:170894-24-3
- Persianone
Catalog No.:BCN7359
CAS No.:170894-20-9
- Cyasterone
Catalog No.:BCN5416
CAS No.:17086-76-9
- Sonepiprazole
Catalog No.:BCC7879
CAS No.:170858-33-0
- PNU 96415E
Catalog No.:BCC7467
CAS No.:170856-41-4
- (RS)-APICA
Catalog No.:BCC6925
CAS No.:170847-18-4
- E4CPG
Catalog No.:BCC6888
CAS No.:170846-89-6
- CHPG
Catalog No.:BCC6910
CAS No.:170846-74-9
- Astressin
Catalog No.:BCC5790
CAS No.:170809-51-5
- H-Ser(tBu)-OMe.HCl
Catalog No.:BCC3033
CAS No.:17114-97-5
- PD 158780
Catalog No.:BCC7434
CAS No.:171179-06-9
- N-Methylquipazine dimaleate
Catalog No.:BCC6697
CAS No.:171205-17-7
- Posaconazole
Catalog No.:BCC1103
CAS No.:171228-49-2
- TAPI-1
Catalog No.:BCC5400
CAS No.:171235-71-5
- (RS)-AMPA hydrobromide
Catalog No.:BCC6926
CAS No.:171259-81-7
- Delphinidin-3-O-arabinoside chloride
Catalog No.:BCN3021
CAS No.:171370-55-1
- Ethyl 4-hydroxyphenylacetate
Catalog No.:BCN3792
CAS No.:17138-28-2
- Calcium Gluceptate
Catalog No.:BCC3743
CAS No.:17140-60-2
- Otophylloside B 4'''-O-alpha-L-cymaropyranoside
Catalog No.:BCN7511
CAS No.:171422-82-5
- Otophylloside B 4'''-O-beta-D-cymaropyranoside
Catalog No.:BCN7522
CAS No.:171422-85-8
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
Acupuncture inhibition of methamphetamine-induced behaviors, dopamine release and hyperthermia in the nucleus accumbens: mediation of group II mGluR.[Pubmed:29363229]
Addict Biol. 2019 Mar;24(2):206-217.
Methamphetamine (METH) increases metabolic neuronal activity in the mesolimbic dopamine (DA) system and mediates the reinforcing effect. To explore the underlying mechanism of acupuncture intervention in reducing METH-induced behaviors, we investigated the effect of acupuncture on locomotor activity, ultrasonic vocalizations, extracellular DA release in the nucleus accumbens (NAcs) using fast-scan cyclic voltammetry and alterations of brain temperature (an indicator of local brain metabolic activity) produced by METH administration. When acupuncture was applied to HT7, but not TE4, both locomotor activity and 50-kHz ultrasonic vocalizations were suppressed in METH-treated rats. Acupuncture at HT7 attenuated the enhancement of electrically stimulated DA release in the NAc of METH-treated rats. Systemic injection of METH produced a sustained increase in NAc temperature, which was reversed by the DA D1 receptor antagonist SCH 23390 or acupuncture at HT7. Acupuncture inhibition of METH-induced NAc temperature was prevented by pre-treatment with a group II metabotropic glutamate receptors (mGluR2/3) antagonist EGLU into the NAc or mimicked by injection of an mGluR2/3 agonist DCG-IV into the NAc. These results suggest that acupuncture reduces extracellular DA release and metabolic neuronal activity in the NAc through activation of mGluR2/3 and suppresses METH-induced affective states and locomotor behavior.
Stimulating ERK/PI3K/NFkappaB signaling pathways upon activation of mGluR2/3 restores OGD-induced impairment in glutamate clearance in astrocytes.[Pubmed:24206109]
Eur J Neurosci. 2014 Jan;39(1):83-96.
We used the oxygen and glucose deprivation (OGD) method in cultured astrocytes as an in vitro ischemic model. We investigated whether activation of group-II metabotropic glutamate receptors (mGluR2/3) can reverse OGD-induced impairment in astrocytic glutamate/aspartate transporter (GLAST) expression and elucidated the signaling pathways involving the GLAST expression. Cultured astrocytes exposed to OGD for 6 h resulted in significant reductions in the GLAST expression and extracellular glutamate clearance. These reductions were effectively restored by mGluR2/3 activation with mGluR2/3 agonists, LY379268 or DCG-IV, after the 6 h OGD insult. These mGluR2/3-mediated restorative effects were inhibited by selective mGluR2/3 antagonists LY341459 or EGLU. The mGluR2/3 activation also induced activations of signaling pathways including extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K) and nuclear transcription factor-kappaB (NFkappaB). These activations were prevented by blocking mGluR2/3 with LY341459, an mGluR2/3 antagonist. Furthermore, blocking ERK, PI3K and NFkappaB signaling pathways with U0126, LY294002 and pyrrolidine dithiocarbamate, respectively, significantly inhibited the mGluR2/3-mediated restorative effects. These results suggest that application of mGluR2/3 agonists after OGD insult can effectively reverse the OGD-reduced expression of GLAST proteins and restore clearance of extracellular glutamate by serially activating ERK/PI3K/NFkappaB signaling pathways in cultured astrocytes.
Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone.[Pubmed:23587885]
J Physiol. 2013 Jun 15;591(12):3081-100.
Oxytocin (OXT) inputs to the dorsal vagal complex (DVC; nucleus of the tractus solitarius (NTS) dorsal motor nucleus of the vagus (DMV) and area postrema) decrease gastric tone and motility. Our first aim was to investigate the mechanism(s) of OXT-induced gastric relaxation. We demonstrated recently that vagal afferent inputs modulate NTS-DMV synapses involved in gastric and pancreatic reflexes via group II metabotropic glutamate receptors (mGluRs). Our second aim was to investigate whether group II mGluRs similarly influence the response of vagal motoneurons to OXT. Microinjection of OXT in the DVC decreased gastric tone in a dose-dependent manner. The OXT-induced gastric relaxation was enhanced following bethanechol and reduced by l-NAME administration, suggesting a nitrergic mechanism of gastroinhibition. DVC application of the group II mGluR antagonist EGLU induced a gastroinhibition that was not dose dependent and shifted the gastric effects of OXT to a cholinergic-mediated mechanism. Evoked and miniature GABAergic synaptic currents between NTS and identified gastric-projecting DMV neurones were not affected by OXT in any neurones tested, unless the brainstem slice was (a) pretreated with EGLU or (b) derived from rats that had earlier received a surgical vagal deafferentation. Conversely, OXT inhibited glutamatergic currents even in naive slices, but their responses were unaffected by EGLU pretreatment. These results suggest that the OXT-induced gastroinhibition is mediated by activation of the NANC pathway. Inhibition of brainstem group II mGluRs, however, uncovers the ability of OXT to modulate GABAergic transmission between the NTS and DMV, resulting in the engagement of an otherwise silent cholinergic vagal neurocircuit.
Anxiolytic properties of Valeriana officinalis in the zebrafish: a possible role for metabotropic glutamate receptors.[Pubmed:22923195]
Planta Med. 2012 Nov;78(16):1719-24.
Valerian extract is used in complementary and alternative medicine for its anxiolytic and sedative properties. Our previous research demonstrated valerian interactions with glutamate receptors. The purpose of this study was to determine if valerian anxiolytic properties are mediated by metabotropic glutamate receptors (mGluR) such as mGluR (1/5) (mGluR I) and mGluR (2/3) (mGluR II). Adult wild-type zebrafish (Danio rerio) prefer the black compartment and avoid the white compartment in the dark/light preference task. Zebrafish exposed to 1 mg/mL of valerian extract or 0.00117 mg/mL valerenic acid increased their residence time in the white side by 84.61 +/- 6.55 % and 58.30 +/- 8.97 %, respectively. LAP3 (mGluR I antagonist) and EGLU (mGluR II antagonist) significantly inhibited the effects of valerian and valerenic acid. These results demonstrated that valerian and valerenic acid have anxiolytic properties in the zebrafish. Moreover, the selective interaction of valerian with mGluR I and II represent an alternative explanation for the anxiolytic properties of this plant and support the role of mGluR in anxiety.
[Involvement of cAMP-PKA pathway in group metabotropic glutamate receptors-mediated regulation of respiratory rhythm from neonatal rat brainstem slice].[Pubmed:21681341]
Sheng Li Xue Bao. 2011 Jun 25;63(3):233-7.
The study aims to identify the role of cAMP-PKA pathway in the group metabotropic glutamate receptors (mGluRs)-mediated regulation of respiratory rhythm from the brainstem slice. Neonatal (aged 0-3 d) Sprague-Dawley rats of either sex were used. The brainstem slice containing the medial region of the nucleus retrofacialis (mNRF) and the hypoglossal nerve rootlets was prepared, and the surgical procedure was performed in the modified Kreb's solution (MKS) with continuous carbogen (95% O2 and 5% CO2) bubbling, and ended in 3 min. Respiratory rhythmical discharge activity (RRDA) of the hypoglossal nerve rootlets was recorded by suction electrode. Eighteen brainstem slice preparations were divided into 3 groups. In group 1, group mGluRs specific antagonist (2S)-alpha-ethylglutamic acid (EGLU) was added into the perfusion solution for 10 min. In group 2, after application of Forskolin for 10 min, washout with MKS, the slice was perfused with Rp-cyclic 3', 5'-hydrogen phosphorothioate adenosine triethylammonium salt (Rp-cAMPS) alone for another 10 min. In group 3, after application of Rp-cAMPS for 10 min, additional EGLU was added into the perfusion for another 10 min. The results showed EGLU shortened respiratory cycle (RC), but the changes of integral amplitude (IA) and inspiratory time (TI) were not statistically significant. Forskolin induced significant decreases in RC, and increased TI, IA. Rp-cAMPS could make the opposite effect compared with the changes of RRDA with Forskolin. The effect of EGLU on the RRDA was inhibited after blocking the cAMP-PKA pathway. Taken together, cAMP-PKA pathway may play an important role in the group mGluRs-mediated regulation of RRDA in the brainstem slice of neonatal rats.
Activity-dependent gamma-aminobutyric acid release controls brain cortical tissue slice metabolism.[Pubmed:21618581]
J Neurosci Res. 2011 Dec;89(12):1935-45.
Vigabatrin (gamma-vinyl-GABA) is an irreversible inhibitor of the enzyme gamma-aminobutyric acid (GABA) transaminase. It has been shown to increase levels of GABA in brain and result in increased release of GABA from nonsynaptic sources following activation. Here, we use a guinea pig cortical tissue slice model to identify the metabolic sequelae of vigabatrin when incubated with tissue slices alone or when the tissue slices were activated by ligands with targeted activating mechanisms. We show that incubation of slices with AMPA, the group II metabotropic glutamate antagonist EGLU [(2S)-alpha-ethylglutamic acid], or the GABA(B) R antagonist CGP 52432 in the presence of vigabatrin produces very similar metabolic profiles, consistent with the large-scale turning off of metabolic activity. This effect is blocked by the GABA(Arho) antagonist TPMPA [(1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid]. Taken together, these results suggest that GABA, released following activation, acts on extrasynaptic receptors consistent with GABA(Arho) and that these receptors act as a kind of "master switch" that is capable of turning off a range of differently induced activities.
Participation of metabotropic glutamate receptors in pentetrazol-induced kindled seizure.[Pubmed:21054350]
Epilepsia. 2011 Jan;52(1):140-50.
PURPOSE: The present study was undertaken to clarify the effects of (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), a metabotropic glutamate receptor (mGluR) 1 antagonist, (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate ((2R,4R)-APDC), a mGluR2/3 agonist, and L-(+)-2-amino-4-phosphonobutyric acid (L-AP4), a mGluR4/8 agonist, on pentetrazol-induced kindled seizures. METHODS: Mice were anesthetized with pentobarbital; the electrodes and guide cannula were chronically implanted into the cortex and lateral ventricle. To induce kindling, pentetrazol at a dose of 40 mg/kg was injected once every 48 h. Behavioral and electroencephalographic seizures were monitored for 20 min following pentetrazol administration. Fully kindled mice were used for pharmacologic studies. RESULTS: Intracerebroventricular injection of AIDA and L-AP4 showed significant inhibitory effects on pentetrazol-induced kindled seizures. In addition, simultaneous use of AIDA and (2R,4R)-APDC or L-AP4 caused more potent inhibition of seizure activities. The inhibitory effect of AIDA on pentetrazol-induced kindled seizures was antagonized by (RS)-3,5-dihydroxyphenylglycine ((RS)-3,5-DHPG), a group I mGluR agonist; (2S)-a-ethylglutamic acid (EGLU), a group II mGluR antagonist; and (RS)-alpha-methyl-4-phosphonophenylglycine (MPPG), a group III mGluR antagonist. On the other hand, the inhibitory effect of L-AP4 was antagonized only by MPPG. DISCUSSION: It is proposed that mGluR1 antagonists and mGluR4/8 agonists show anticonvulsive effects on pentetrazol-induced kindled seizures. Furthermore, it is also proposed that the simultaneous use of an mGluR1 antagonist and an mGluR2/3 or mGluR4/8 agonist is a potential novel therapeutic strategy in epileptic disorders.
[Group II metabotropic glutamate receptors is involved in the modulation of respiratory rhythmical discharge activity in neonatal rat medullary brain slices].[Pubmed:20813672]
Nan Fang Yi Ke Da Xue Xue Bao. 2010 Aug;30(8):1813-6.
OBJECTIVE: To explore the role of group II metabotropic glutamate receptors in the modulation of basic respiratory rhythm. METHODS: Neonatal (0-3 days) SD rats of either sex were used. The medulla oblongata brain slice containing the medial region of the nucleus retrofacialis (mNRF) and the hypoglossal nerve rootlets was prepared, and the surgical procedure was performed in the modified Kreb's solution (MKS) with continuous carbogen (95% O2 and 5% CO2) within 3 min. The brain slices were quickly transferred to a recording chamber and continuously perfused with oxygen-saturated MKS at a rate of 4-6 ml/min at 27-29 degrees celsius. Eighteen medulla oblongata slices were divided into 3 groups and treated for 10 min with group II metabotropic glutamate receptor-specific agonist 2R,4R-4-aminopyrrolidine-2,4-dicarboxylate (APDC) (at concentrations of 10, 20, 50 micromol/L), group II metabotropic glutamate receptor antagonist (2S)-alpha-ethylglutamic acid (EGLU) (300 micromol/L), or APDC (50 micromol/L)+EGLU (300 micromol/L) after a 10 min APDC (50 micromol/L) application. Respiratory rhythmical discharge activity (RRDA) of the rootlets of the hypoglossal nerve was recorded by suction electrodes. RESULTS: APDC produced a dose-dependent inhibitory effect on the RRDA, prolonging the respiratory cycle and expiratory time and decreasing the integral amplitude and inspiratory time. EGLU induced a significant decrease in the respiratory cycle and expiratory time. The effect of APDC on the respiratory rhythm was partially reversed by the application of APDC+EGLU. CONCLUSION: Group II metabotropic glutamate receptors are probably involved in the modulation of the RRDA in isolated neonatal rat brainstem slice.
[The role of group II and III metabotropic glutamate receptors in modulation of miniature synaptic activity in frog spinal cord motoneurons].[Pubmed:18959186]
Tsitologiia. 2008;50(9):747-56.
The results of present work demonstrate significant modulating effects mediated by group II and III mGluRs on miniature postsynaptic potentials (mPSP) of the frog spinal motoneurons. The mode of group II and III mGluRs ligands influences, i. e. the changes in the mPSPs average frequency without significant changes in their average amplitude, suggests the presynaptic mechanism of modulation by the change in transmitter release. Selective antagonists of group II and III mGluRs (EGLU and MAP4) increased the average frequency of mPSPs by 52.8 +/- 30.2% (in 4 of 6 motoneurons) and by 54.7 +/- 23.7% (in all 7 motoneurons), respectively. Application of the group III mGluRs agonist LAP4 decreased the mPSPs frequency by 21.8 +/- 5.2% in 3 of 5 motoneurons. The efficiency of the antagonist usage and comparative low efficiency of the agonist suggest that presynaptic mGluRs at motoneuronal synapses under normal condition possess some level of tonic activity. The lack of group II mGluR antagonist effect on some motoneurons appears to be explained by specific localization of the group II mGluRs in preterminal area which is distant from the transmitter release site. The hetero-receptor modulation of pharmacologically isolated inhibitory miniature activity and its glycine- and GABAergic fractions by group III mGluRs was investigated. MAP4 application has been shown to increase the glycine-mediated mlPSPs frequency more than GABA-mediated mlPSPs frequency: in average by 97.6 +/- 20.7% (n = 7) and 54.6 +/- 20.8% (n = 5), respectively. This difference may be due to the segregation of the postsynaptic glycine- and GABA-receptors. The preliminary examination of the convergence of the presynaptic mGluRs and metabotropic GABA(B) receptors influences on GABA-mediated IPSPs was undertaken. It has been shown that presynaptic GABA(B) receptors are tonically active under normal condition. Under condition of GABA(B) receptor blockage by phaclofen, the application of group III mGluR agonist L-AP4 elicited typical effect which was completely taken off by subsequent application of the group III mGluRs antagonist MAP4. This result is in accordance with the assumption that the effects mediated by GABA(B) receptors and mGluRs are independent.
A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory.[Pubmed:18722513]
Neuroscience. 2009 Jan 12;158(1):149-58.
Activity-dependent and sustained alterations in synaptic efficacy are widely regarded as the cellular correlates underlying learning and memory. Metabotropic glutamate receptors (mGluRs) are intrinsically involved in both hippocampal synaptic plasticity and spatial learning. Group II mGluRs are required for persistent hippocampal long-term depression (LTD), but are not required for long-term potentiation (LTP) in the hippocampal CA1 region in vivo. The role of these receptors in spatial learning, and in synaptic plasticity in the dentate gyrus in vivo has not yet been the subject of close scrutiny. We investigated the effects of group II mGluR antagonism on LTP and LTD in the adult rat, at medial perforant path-dentate gyrus synapses, and on spatial learning in the eight-arm radial maze. Daily application of the group 2 mGluR antagonist (2S)-alpha-ethylglutamic acid (EGLU) resulted in impairment of long-term (reference) memory with effects becoming apparent 6 days after training and drug-treatment began. Short-term (working) memory was unaffected throughout the 10-day study. Acute injection of EGLU did not affect either LTD or LTP in the dentate gyrus in vivo. Following six daily applications of EGLU a clear impairment of LTD but not LTP was apparent however. These data support that prolonged antagonism of group II mGluRs results in an impairment of LTD that parallels the appearance of spatial memory deficits arising from group II mGluR antagonism. These findings support the importance of group II mGluRs for spatial memory formation and offer a further link between LTD and the encoding of spatial information in the hippocampus.
Activation of group I metabotropic glutamate receptors induces long-term depression in the hippocampal CA1 region of adult rats in vitro.[Pubmed:18602428]
Neurosci Res. 2008 Sep;62(1):43-50.
Previous studies have implicated that long-term depression (LTD) was developmentally regulated since LTD can be readily induced by low frequency stimulation (LFS) in acute hippocampal slices prepared from juvenile but not adult animals. Here, we have examined the LTD induced by LFS (1Hz, 900 pulses) paired with a certain pattern at the Schaffer collateral-CAl synapse in adult hippocampal slices. We found that, in the 90-day-old rat hippocampus, LTD could be induced reliably by LFS paired with stronger stimulus intensity than that used during baseline recording. However, this synaptic depression could be completely abolished by application of metabotropic glutamate receptor (mGluR) antagonist (S)-amethyl-4-carboxyphenylglycine (MCPG) which had no effect on that induced by the same protocol in the 16-day-old rat hippocampus. Furthermore, preincubation with group I mGluR antagonist, 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and (S)-2-methyl-4-carboxyphenylglycine (LY367385), also completely prevented the LFS-induced LTD. In contrast, group II mGluR antagonist (2S)-a-ethylglutamic acid (EGLU), N-methyl-d-aspartate (NMDA) receptor antagonist APV and voltage-gated calcium channel antagonist nimodipine had no effect on the LFS-induced LTD. Taken together, these observations suggest that LFS paired with strong stimulus strength can efficiently induce group I mGluR-dependent LTD in the adult hippocampal CA1 region, proving insight into the functional significance of hippocampal mGluR-mediated LTD in learning and memory.
Blockade of group II, but not group I, mGluRs in the rat nucleus accumbens inhibits the expression of conditioned hyperactivity in an amphetamine-associated environment.[Pubmed:18433894]
Behav Brain Res. 2008 Aug 5;191(1):62-6.
Environmental stimuli associated with amphetamine (AMPH) can elicit conditioned locomotion in rats, and the nucleus accumbens (NAcc) is known to be important in this process. This study examined the contribution of metabotropic glutamate receptors (mGluRs) in the NAcc to the expression of conditioned locomotion in an AMPH-associated environment. Rats in different groups were administered injections in five 3-day blocks: Paired, AMPH (1.0mg/kg, IP) in locomotor activity boxes on day 1 and saline in their home cages on day 2; Unpaired, saline in the activity boxes on day 1 and AMPH in their home cages on day 2; or Control, saline in both environments. No injections were administered on day 3 of each block. One week after the last conditioning block, all rats were tested for their conditioned locomotor response in the activity boxes for 1h following an IP saline injection. In Paired rats, this injection was preceded by a bilateral microinjection into the NAcc of saline, the group I mGluR antagonist, AIDA (0.5, 5.0 nmol/side), or the group II mGluR antagonist, EGLU (0.5, 5.0 nmol/side). Unpaired and Control rats received NAcc saline. As expected, Paired rats showed both increased locomotor activity and rearing compared to rats in either the Unpaired or Control groups. However, the expression of this conditioned hyper-locomotion was dose-dependently inhibited by NAcc EGLU, but not by AIDA. These results suggest that activation of group II, but not of group I, mGluRs in the NAcc contributes to the expression of conditioned locomotion in an environment associated with amphetamine.
Modulation of a spinal locomotor network by metabotropic glutamate receptors.[Pubmed:17894819]
Eur J Neurosci. 2007 Oct;26(8):2257-68.
We have explored the potential involvement of the three main classes of metabotropic glutamate receptor in the modulation of a spinal locomotor network using tadpoles of the anuran amphibian Xenopus laevis. Selective activation of group I receptors in Xenopus embryos and young larvae using the general group I agonist DHPG [(S)-3,5-dihyroxyphenylglycine] significantly increased the frequency of swimming and the number of spontaneously occurring swimming episodes, as monitored by extracellular recordings from ventral roots. Group I receptor activation was without significant effect on the duration or amplitude of motor bursts, the duration of swimming episodes, or the head-to-tail delay in the propagation of swimming activity. Activation of either group II or group III receptors, however, following bath applications of the specific agonists APDC [(2R,4R)-aminopyrrolidine-2,4-dicarboxylic acid] and L-AP4 (L-2-amino-4-phosphonobutanoate), respectively, produced a net inhibitory effect on many of the parameters of fictive swimming at both developmental stages, including a reduction in swimming frequency and episode duration, along with a significant reduction in motor burst amplitude and duration in larval animals only. Applications of selective antagonists provide evidence for activation of all three groups during swimming. The group II and III antagonists EGLU (1-ethyl-2-benzimidazolinone) and MAP4 [(S)-2-amino-2-methyl-4-phosphonobutanoate], respectively, increased, while group I antagonists, CPCCOEt and MPEP, decreased swim frequency. Our findings thus provide evidence for the presence and endogenous activation of three classes of metabotropic glutamate receptor which may function as an intrinsic modulatory control system during fictive swimming in Xenopus tadpoles.
Potent antagonists at the L-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord.[Pubmed:9121605]
Neuropharmacology. 1996;35(8):1029-35.
In this report we describe the actions of two novel compounds, (RS)-alpha-cyclopropyl-4-phosphonophenylglycine (CPPG) and (S)-alpha-ethylglutamate (EGLU), which are potent antagonists at two types of presynaptic metabotropic glutamate (mGlu) receptors in the neonatal rat spinal cord. Selective activation of these receptors by L-2-amino-4-phosphonobutyrate (L-AP4) or (1S,3S)-1-aminocyclopentane-1,3-dicarboxylic acid ((1S,3S)-ACPD) results in the depression of the monosynaptic component of the dorsal root-evoked ventral root potential (DR-VRP). CPPG produces rightward parallel shifts of the dose-response curves for both L-AP4- and (1S,3S)-ACPD, with Schild slope in each case close to unity, consistent with a competitive mechanism of antagonism. CPPG is the most potent antagonist yet described for both L-AP4- and (1S,3S)-ACPD-sensitive presynaptic mGlu receptors but displays a 30-fold selectivity for the L-AP4-sensitive receptor over the (1S,3S)-ACPD-sensitive receptor (KD values 1.7 microM and 53 microM, respectively). EGLU, on the other hand, is selective for the (1S,3S)-ACPD-sensitive receptor, displaying little or no activity at the L-AP4-sensitive site. EGLU produces a rightward parallel shift of the dose-response curve to (1S,3S)-ACPD, with Schild slope close to unity, again indicative of a competitive mode of antagonism (KD 66 microM). Both CPPG and EGLU displayed only weak or no antagonist activity at postsynaptic metabotropic and ionotropic glutamate receptors.


