AstressinPotent CRF receptor antagonist CAS# 170809-51-5 |
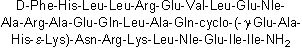
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
- Quizartinib (AC220)
Catalog No.:BCC2548
CAS No.:950769-58-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure
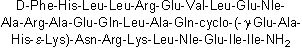
3D structure
| Cas No. | 170809-51-5 | SDF | Download SDF |
| PubChem ID | 16133798 | Appearance | Powder |
| Formula | C161H269N49O42 | M.Wt | 3563.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | [D-Phe<sup>12</sup>, Nle<sup>21,38</sup>, Glu<sup>30</sup>, Lys<sup>33</sup>]-CRF (12-41) | ||
| Solubility | H2O Peptide Solubility and Storage Guidelines: 1. Calculate the length of the peptide. 2. Calculate the overall charge of the entire peptide according to the following table: 3. Recommended solution: | ||
| Sequence | FHLLREVLEXARAEQLAQEAHKNRKLXEII (Modifications: Phe-1 = D-Phe, X = Nle, Glu-19 = γ-Glu, Lys-22 = ε-Lys, Cyclized = Glu-19 -Lys-22, Ile-31 = C-terminal amide) | ||
| SMILES | CCCCC(C(=O)NC(C)C(=O)NC(CCCNC(=N)N)C(=O)NC(C)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)N)C(=O)NC(CC(C)C)C(=O)NC(C)C(=O)NC(CCC(=O)N)C(=O)NC1CCC(=O)NCCCCC(NC(=O)C(NC(=O)C(NC1=O)C)CC2=CNC=N2)C(=O)NC(CC(=O)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCCN)C(=O)NC(CC(C)C)C(=O)NC(CCCC)C(=O)NC(CCC(=O)O)C(=O)NC(C(C)CC)C(=O)NC(C(C)CC)C(=O)N)NC(=O)C(CCC(=O)O)NC(=O)C(CC(C)C)NC(=O)C(C(C)C)NC(=O)C(CCC(=O)O)NC(=O)C(CCCNC(=N)N)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC3=CNC=N3)NC(=O)C(CC4=CC=CC=C4)N | ||
| Standard InChIKey | HPYIIXJJVYSMCV-MGDXKYBTSA-N | ||
| Standard InChI | InChI=1S/C161H269N49O42/c1-23-27-41-96(189-145(239)107(52-59-123(217)218)199-152(246)114(71-84(13)14)207-157(251)126(85(15)16)208-147(241)108(53-60-124(219)220)197-140(234)102(47-38-66-179-161(172)173)193-151(245)112(69-82(9)10)204-153(247)113(70-83(11)12)205-155(249)116(74-94-77-175-79-181-94)201-134(228)95(163)72-92-39-30-29-31-40-92)135(229)182-88(19)130(224)186-100(45-36-64-177-159(168)169)136(230)183-89(20)131(225)188-106(51-58-122(215)216)144(238)195-104(49-56-119(165)212)146(240)202-110(67-80(5)6)149(243)185-90(21)132(226)187-103(48-55-118(164)211)143(237)196-105-50-57-121(214)176-63-35-33-44-99(192-154(248)115(73-93-76-174-78-180-93)200-133(227)91(22)184-137(105)231)142(236)206-117(75-120(166)213)156(250)194-101(46-37-65-178-160(170)171)139(233)190-98(43-32-34-62-162)141(235)203-111(68-81(7)8)150(244)191-97(42-28-24-2)138(232)198-109(54-61-125(221)222)148(242)210-128(87(18)26-4)158(252)209-127(129(167)223)86(17)25-3/h29-31,39-40,76-91,95-117,126-128H,23-28,32-38,41-75,162-163H2,1-22H3,(H2,164,211)(H2,165,212)(H2,166,213)(H2,167,223)(H,174,180)(H,175,181)(H,176,214)(H,182,229)(H,183,230)(H,184,231)(H,185,243)(H,186,224)(H,187,226)(H,188,225)(H,189,239)(H,190,233)(H,191,244)(H,192,248)(H,193,245)(H,194,250)(H,195,238)(H,196,237)(H,197,234)(H,198,232)(H,199,246)(H,200,227)(H,201,228)(H,202,240)(H,203,235)(H,204,247)(H,205,249)(H,206,236)(H,207,251)(H,208,241)(H,209,252)(H,210,242)(H,215,216)(H,217,218)(H,219,220)(H,221,222)(H4,168,169,177)(H4,170,171,178)(H4,172,173,179)/t86-,87-,88-,89-,90-,91-,95+,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,126-,127-,128-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent corticotropin-releasing factor (CRF) receptor antagonist (Ki values are 2, 1.5 and 1 nM at CRF1, CRF2α and CRF2β). Reduces ACTH secretion, blocks delayed gastric emptying and is neuroprotective in vivo. |

Astressin Dilution Calculator

Astressin Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Astressin is a potent corticotropin releasing factor (CRF) antagonist. Sequence: d-Phe-His-Leu-Leu-Arg-Glu-Val-Leu-Glu-NLE-Ala-Arg-Ala-Glu-Gln-Leu-Ala-Gln-Glu-Ala-His-Lys-Asn-Arg-Lys-Leu-NLE-Glu-Ile-Ile-NH2.
In Vitro:Astressin has low affinity for the CRF binding protein and high affinity (Ki=2 nM) for the cloned pituitary receptor. Astressin shows high affinity for cloned human CRF-RA1 stably expressed in CHO cells and high potency to inhibit ACTH secretion[1].
In Vivo:Astressin is significantly more potent than any previously tested antagonist in reducing hypophyseal corticotropin (ACTH) secretion in stressed or adrenalectomized rats. Low doses of astressin (30 μg and 100 μg per kg) administered i.v. still produce a significant decrease in ACTH levels at 45 and 90 min, respectively[1]. Astressin significantly reverses the anxiogenic-like response induced by both social stress and ICV rat/humanCRF (r/hCRF) on the elevated plus-maze, but fails to block the effects of r/hCRF-induced locomotor activity in a familiar environment[2]. Intracerebroventricular infusion of the peptide both 30 min before and 10 min after seizures decreases damage in some hippocampal cell fields by as much as 84%, a magnitude of protection greater than reported for other CRF antagonists against other models of necrotic neuronal injury. Astressin protects even if administered only 10 min following excitotoxin exposure[3].
References:
[1]. Gulyas J, et al. Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10575-9.
[2]. Spina MG, et al. Behavioral effects of central administration of the novel CRF antagonist astressin in rats. Neuropsychopharmacology. 2000 Mar;22(3):230-9.
[3]. Maecker H, et al. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997 Jan 2;744(1):166-70.
- Trityl candesartan cilexetil
Catalog No.:BCC9188
CAS No.:170791-09-0
- Aprepitant
Catalog No.:BCC1101
CAS No.:170729-80-3
- 11-Deoxymogroside V
Catalog No.:BCN8143
CAS No.:1707161-17-8
- Nociceptin
Catalog No.:BCC5686
CAS No.:170713-75-4
- 6beta-Hydroxyhispanone
Catalog No.:BCN7453
CAS No.:170711-93-0
- Bindone
Catalog No.:BCC8877
CAS No.:1707-95-5
- D-Mannitol diacetonide
Catalog No.:BCC8951
CAS No.:1707-77-3
- α-Conotoxin EI
Catalog No.:BCC5979
CAS No.:170663-33-9
- Fmoc-D-Abu-OH
Catalog No.:BCC3203
CAS No.:170642-27-0
- YC 1
Catalog No.:BCC7912
CAS No.:170632-47-0
- Oteromycin
Catalog No.:BCN1849
CAS No.:170591-45-4
- SC 236
Catalog No.:BCC7809
CAS No.:170569-86-5
- CHPG
Catalog No.:BCC6910
CAS No.:170846-74-9
- E4CPG
Catalog No.:BCC6888
CAS No.:170846-89-6
- (RS)-APICA
Catalog No.:BCC6925
CAS No.:170847-18-4
- PNU 96415E
Catalog No.:BCC7467
CAS No.:170856-41-4
- Sonepiprazole
Catalog No.:BCC7879
CAS No.:170858-33-0
- Cyasterone
Catalog No.:BCN5416
CAS No.:17086-76-9
- Persianone
Catalog No.:BCN7359
CAS No.:170894-20-9
- Aburatubolactam A
Catalog No.:BCN1821
CAS No.:170894-24-3
- Donitriptan hydrochloride
Catalog No.:BCC7742
CAS No.:170911-68-9
- Dihydroactinidiolide
Catalog No.:BCN6890
CAS No.:17092-92-1
- Tetrindole mesylate
Catalog No.:BCC6763
CAS No.:170964-68-8
- EGLU
Catalog No.:BCC6871
CAS No.:170984-72-2
Increased ghrelin sensitivity and calorie consumption in subordinate monkeys is affected by short-term astressin B administration.[Pubmed:20981508]
Endocrine. 2010 Oct;38(2):227-34.
Animals chronically exposed to stressors with access to diets high in fat and sugar consume and prefer these diets, a result consistent with the association between stress and comfort food ingestion in humans. As social subordination in rhesus monkeys provides an ethologically relevant translational model of psychosocial stress, we tested the hypothesis that differences in food intake between dominant and subordinate female monkeys are due to corticotropin-releasing hormone-(CRH) induced alteration in sensitivity to ghrelin, a potent orexigenic signal. We assessed food intake of animals given a choice between a low (LCD) and high calorie diet (HCD) in response to 4-day treatment with the CRH receptor antagonist, Astressin B, and to an acute treatment of ghrelin. Ghrelin stimulated intake of LCD in subordinates but did not further increase consumption of HCD, whereas ghrelin decreased LCD consumption without affecting HCD intake in dominant females. Astressin B decreased cortisol levels and increased preference for and intake of the HCD in subordinates and decreased calorie intake and HCD preference in dominant animals. These results suggest that increased caloric intake by subordinates may, in part, be explained by a greater sensitivity to postprandial increases in ghrelin and that CRH receptor antagonism leading to a decrease in cortisol has mixed effects on food choice depending on an individual's stress background.
CRF receptor antagonist astressin-B reverses and prevents alopecia in CRF over-expressing mice.[Pubmed:21359208]
PLoS One. 2011 Feb 16;6(2):e16377.
Corticotropin-releasing factor (CRF) signaling pathways are involved in the stress response, and there is growing evidence supporting hair growth inhibition of murine hair follicle in vivo upon stress exposure. We investigated whether the blockade of CRF receptors influences the development of hair loss in CRF over-expressing (OE)-mice that display phenotypes of Cushing's syndrome and chronic stress, including alopecia. The non-selective CRF receptors antagonist, Astressin-B (5 microg/mouse) injected peripherally once a day for 5 days in 4-9 months old CRF-OE alopecic mice induced pigmentation and hair re-growth that was largely retained for over 4 months. In young CRF-OE mice, Astressin-B prevented the development of alopecia that occurred in saline-treated mice. Histological examination indicated that alopecic CRF-OE mice had hair follicle atrophy and that Astressin-B revived the hair follicle from the telogen to anagen phase. However, Astressin-B did not show any effect on the elevated plasma corticosterone levels and the increased weights of adrenal glands and visceral fat in CRF-OE mice. The selective CRF(2) receptor antagonist, Astressin(2)-B had moderate effect on pigmentation, but not on hair re-growth. The commercial drug for alopecia, minoxidil only showed partial effect on hair re-growth. These data support the existence of a key molecular switching mechanism triggered by blocking peripheral CRF receptors with an antagonist to reset hair growth in a mouse model of alopecia associated with chronic stress.
Astressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkey.[Pubmed:18063681]
Endocrinology. 2008 Mar;149(3):869-74.
Administration of ghrelin, a key peptide in the regulation of energy homeostasis, has been shown to decrease LH pulse frequency while concomitantly elevating cortisol levels. Because increased endogenous CRH release in stress is associated with an inhibition of reproductive function, we have tested here whether the pulsatile LH decrease after ghrelin may reflect an activated hypothalamic-pituitary-adrenal axis and be prevented by a CRH antagonist. After a 3-h baseline LH pulse frequency monitoring, five adult ovariectomized rhesus monkeys received a 5-h saline (protocol 1) or ghrelin (100-microg bolus followed by 100 microg/h, protocol 2) infusion. In protocols 3 and 4, animals were given Astressin B, a nonspecific CRH receptor antagonist (0.45 mg/kg im) 90 min before ghrelin or saline infusion. Blood samples were taken every 15 min for LH measurements, whereas cortisol and GH were measured every 45 min. Mean LH pulse frequency during the 5-h ghrelin infusion was significantly lower than in all other treatments (P < 0.05) and when compared with the baseline period (P < 0.05). Pretreatment with Astressin B prevented the decrease. Ghrelin stimulated cortisol and GH secretion, whereas Astressin B pretreatment prevented the cortisol, but not the GH, release. Our data indicate that CRH release mediates the inhibitory effect of ghrelin on LH pulse frequency and suggest that the inhibitory impact of an insufficient energy balance on reproductive function may in part be mediated by the hypothalamic-pituitary-adrenal axis.
Effects of acidic-astressin and ovine-CRF microinfusions into the ventral hippocampus on defensive behaviors in rats.[Pubmed:19269291]
Horm Behav. 2009 Jun;56(1):35-43.
This study investigated a possible role for ventral hippocampal corticotropin-releasing factor (CRF) in modulating both unconditioned and conditioned defensive behaviors by examining the effects of pre-training ventral hippocampal ovine-CRF (oCRF) or acidic-Astressin ([Glu(11,16)]Ast) microinfusions in male Long-Evans hooded rats exposed to various threat stimuli including the elevated plus-maze (EPM) (oCRF), cat odor (oCRF and [Glu(11,16)]Ast) and a live cat ([Glu(11,16)]Ast). Unconditioned defensive behaviors were assessed during threat exposure, while conditioned defensive behaviors were assessed in each predator context 24 h after the initial threat encounter. Pre-training infusions of the CRF(1) and CRF(2) receptor agonist oCRF significantly increased defensive behaviors during both the unconditioned and conditioned components of the cat odor test, as well as exposure to the EPM. In contrast to the behavioral effects of oCRF microinfusions, the CRF(1) and CRF(2) receptor antagonist [Glu(11,16)]Ast significantly decreased defensive behaviors during exposure to cat odor, while producing no discernible effects following a second injection in the cat exposure test. During conditioned test trials, pre-training infusions of [Glu(11,16)]Ast also significantly reduced defensive behaviors during re-exposure to both predator contexts. These results suggest a specific role for ventral hippocampal CRF receptors in modulating anxiety-like behaviors in several ethologically relevant animal models of defense.
Peripheral injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks peripheral CRF- and abdominal surgery-induced delayed gastric emptying in rats.[Pubmed:10411571]
J Pharmacol Exp Ther. 1999 Aug;290(2):629-34.
The effect of the corticotropin-releasing factor (CRF) receptor antagonists Astressin and D-Phe CRF(12-41) injected i.v. on CRF-induced delayed gastric emptying (GE) was investigated in conscious rats. Gastric transit was assessed by the recovery of methyl cellulose/phenol red solution 20 min after its intragastric administration. The 55% inhibition of GE induced by CRF (0.6 microgram i.v.) was antagonized by 87 and 100% by i.v. Astressin at 3 and 10 microgram, respectively, and by 68 and 64% by i.v. D-Phe CRF(12-41) at 10 and 20 microgram, respectively. CRF (0.6 microgram)-injected intracisternally (i.c.) induced 68% reduction of GE was not modified by i.v. Astressin (10 microgram) whereas i.c. Astressin (3 or 10 microgram) blocked by 58 and 100%, respectively, i.v. CRF inhibitory action. Abdominal surgery with cecal manipulation reduced GE to 7.1 +/- 3.1 and 27.5 +/- 3.3% at 30 and 180 min postsurgery, respectively, compared with 40.3 +/- 4.3 and 59.5 +/- 2.9% at similar times after anesthesia alone. Astressin (3 microgram i.v.) completely and D-Phe CRF(12-41) (20 microgram i.v.) partially (60%) blocked surgery-induced gastric stasis observed at 30 or 180 min. The CRF antagonists alone (i.v. or i.c.) had no effect on basal GE. These data indicate that CRF acts in the brain and periphery to inhibit GE through receptor-mediated interaction and that peripheral CRF is involved in acute postoperative gastric ileus; Astressin is a potent peripheral antagonist of CRF when injected i.v. whereas i.c. doses >/=3 microgram exert dual central and peripheral blockade of CRF action on gastric transit.
Corticotropin releasing factor receptors and their ligand family.[Pubmed:10816663]
Ann N Y Acad Sci. 1999 Oct 20;885:312-28.
The CRF receptors belong to the VIP/GRF/PTH family of G-protein coupled receptors whose actions are mediated through activation of adenylate cyclase. Two CRF receptors, encoded by distinct genes, CRF-R1 and CRF-R2, and that can exist in two alternatively spliced forms, have been cloned. The type-1 receptor is expressed in many areas of the rodent brain, as well as in the pituitary, gonads, and skin. In the rodent, one splice variant of the type-2 receptor, CRF-R2 alpha, is expressed mainly in the brain, whereas the other variant, CRF-R2 beta, is found not only in the CNS, but also in cardiac and skeletal muscle, epididymis, and the gastrointestinal tract. The poor correlation between the sites of expression of CRF-R2 and CRF, as well as the relatively low affinity of CRF for CRF-R2, suggested the presence of another ligand, whose existence was confirmed in our cloning of urocortin. This CRF-like peptide is found not only in brain, but also in peripheral sites, such as lymphocytes. The broad tissue distribution of CRF receptors and their ligands underscores the important role of this system in maintenance of homeostasis. Functional studies of the two receptor types reveal differences in the specificity for CRF and related ligands. On the basis of its greater affinity for urocortin, in comparison with CRF, as well as its brain distribution, CRF-R2 may be the cognate receptor for urocortin. Mutagenesis studies of CRF receptors directed toward understanding the basis for their specificity, provide insight into the structural determinants for hormone-receptor recognition and signal transduction.
Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure.[Pubmed:9030428]
Brain Res. 1997 Jan 2;744(1):166-70.
Corticotropin-releasing factor (CRF), the principle hypothalamic regulator of the adrenocortical axis, also functions as a neurotransmitter. In this latter role, CRF causes electrophysiological activation and epileptiform activity in various brain regions. That finding, coupled with the observation that CRF mRNA is induced in endangered brain regions following necrotic insults, suggests that the peptide might contribute to necrotic neuron loss. Supporting that, a number of studies have shown that CRF antagonists decrease ischemic or excitotoxic damage to neurons. In the present report, we demonstrate the considerable neuroprotective potential of a novel and potent CRF antagonist, Astressin, against kainic acid-induced excitotoxic seizures. Intracerebroventricular infusion of the peptide both 30 min before and 10 min after seizures decreased damage in some hippocampal cell fields by as much as 84%, a magnitude of protection greater than reported for other CRF antagonists against other models of necrotic neuronal injury. Administration of Astressin was done against both local microinfusion (0.035 microgram) or systemic infusion (10 mg/kg body weight) of the excitotoxin; furthermore, the peptide protected even if administered only 10 min following excitotoxin exposure. This fulfills a critical prerequisite for any eventual therapeutic use of CRF antagonists, namely that they need not be administered in anticipation of a neurological insult.
Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor.[Pubmed:7479843]
Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10575-9.
Predictive methods, physicochemical measurements, and structure activity relationship studies suggest that corticotropin-releasing factor (CRF; corticoliberin), its family members, and competitive antagonists (resulting from N-terminal deletions) usually assume an alpha-helical conformation when interacting with the CRF receptor(s). To test this hypothesis further, we have scanned the whole sequence of the CRF antagonist [D-Phe12,Nle21,38]r/hCRF-(12-41) (r/hCRF, rat/human CRF; Nle, norleucine) with an i-(i + 3) bridge consisting of the Glu-Xaa-Xaa-Lys scaffold. We have found Astressin [cyclo(30-33)[D-Phe12,Nle21,38,Glu30,Lys33]r/ hCRF(12-41)] to be approximately 30 times more potent than [D-Phe12,Nle21,38]r/hCRF-(12-41), our present standard, and 300 times more potent than the corresponding linear analog in an in vitro pituitary cell culture assay. Astressin has low affinity for the CRF binding protein and high affinity (Ki = 2 nM) for the cloned pituitary receptor. Radioiodinated [D-125I-Tyr12]Astressin was found to be a reliable ligand for binding assays. In vivo, Astressin is significantly more potent than any previously tested antagonist in reducing hypophyseal corticotropin (ACTH) secretion in stressed or adrenalectomized rats. The cyclo(30-33)[Ac-Pro4,D-Phe12,Nle21,38,Glu30,Lys33++ +]r/hCRF-(4-41) agonist and its linear analog are nearly equipotent, while the antagonist Astressin and its linear form vary greatly in their potencies. This suggests that the lactam cyclization reinstates a structural constraint in the antagonists that is normally induced by the N terminus of the agonist.


