Tenofovir hydrateReverse transcriptase inhibitor CAS# 206184-49-8 |
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Quality Control & MSDS
Number of papers citing our products

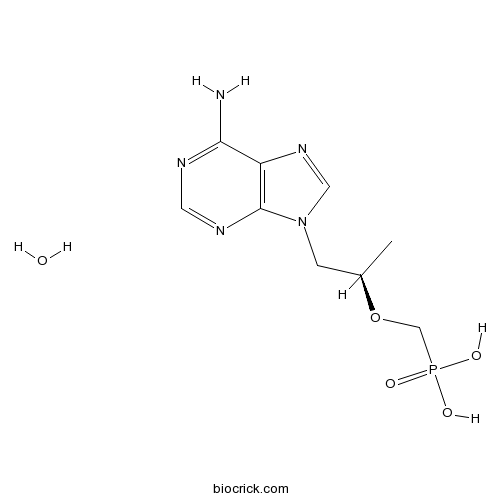
Chemical structure

3D structure
| Cas No. | 206184-49-8 | SDF | Download SDF |
| PubChem ID | 21146529 | Appearance | Powder |
| Formula | C9H16N5O5P | M.Wt | 305.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GS 1278 hydrate; PMPA hydrate; TDF hydrate | ||
| Solubility | DMSO : ≥ 6 mg/mL (19.66 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid;hydrate | ||
| SMILES | CC(CN1C=NC2=C1N=CN=C2N)OCP(=O)(O)O.O | ||
| Standard InChIKey | PINIEAOMWQJGBW-FYZOBXCZSA-N | ||
| Standard InChI | InChI=1S/C9H14N5O4P.H2O/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14;/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17);1H2/t6-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tenofovir hydrate is a nucleotide reverse transcriptase inhibitor to treat HIV and chronic Hepatitis B.In Vitro:Tenofovir shows cytotoxic effects on cell viability in HK-2 cells, with IC50 values of 9.21 and 2.77 μM at 48 and 72 h in MTT assay, respectively. Tenofovir diminishes ATP levels in HK-2 cells. Tenofovir (3.0 to 28.8 μM) increases oxidative stress and protein carbonylation in HK-2 cells. Furthermore, Tenofovir induces apoptosis in HK-2 cells, and that apoptosis is induced via mitochondrial damage[1]. Tenofovir and M48U1 formulated in 0.25% HEC each inhibits the replication of both R5-tropic HIV-1BaL and X4-tropic HIV-1IIIb in activated PBMCs, and inhibits several laboratory strains and patient-derived HIV-1 isolates. The combined formulation of M48U1 and tenofovir in 0.25% HEC exhibits synergistic antiretroviral activity against infection with R5-tropic HIV-1BaL, and is not toxic to PBMCs[2].In Vivo:Tenofovir Disoproxil Fumarate (20, 50, 140, or 300 mg/kg) administered to BLT mice, shows dose dependent activity during vaginal HIV challenge in BLT humanized mice. Tenofovir Disoproxil Fumarate (50, 140, 300 mg/kg) significantly reduces HIV transmission in BLT mice[3]. Tenofovir Disoproxil Fumarate (0.5, 1.5, or 5.0 mg/kg/day, p.o.) induces a dose-dependent decline in serum viremia in woodchucks chronically infected with WHV. Tenofovir Disoproxil Fumarate administration is safe and effective in the woodchuck model of chronic HBV infection[4]. References: | |||||

Tenofovir hydrate Dilution Calculator

Tenofovir hydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2762 mL | 16.3811 mL | 32.7622 mL | 65.5244 mL | 81.9054 mL |
| 5 mM | 0.6552 mL | 3.2762 mL | 6.5524 mL | 13.1049 mL | 16.3811 mL |
| 10 mM | 0.3276 mL | 1.6381 mL | 3.2762 mL | 6.5524 mL | 8.1905 mL |
| 50 mM | 0.0655 mL | 0.3276 mL | 0.6552 mL | 1.3105 mL | 1.6381 mL |
| 100 mM | 0.0328 mL | 0.1638 mL | 0.3276 mL | 0.6552 mL | 0.8191 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tenofovir hydrate is an inhibitor of reverse transcriptase used for the treatment of the human immunodeficiency virus 1(HIV-1) and hepatitis B [1].
Tenofovir hydrate is an antiviral pro-drug and the class of nucleoside reverse transcriptase inhibitor. In addition, Tenofovir hydrate has been reported to have a dependent relation between intracellular the drug concentrations and prevent function of HIV-1infection with EC50 values of 29 fmol/106, 40 fmol/106 , 77 fmol/106 and 411 fmol/106 cells for inoculum size 1, 5, 20 and 100 respectively. And the EC90 values of tenofovir hydrate are 267 fmol/106, 348 fmol/106, 640 fmol/106 and 2866 fmol/106 cells for virus inoculums size 1, 5, 20 and 100, respectively [1].
References:
[1] Duwal S1, Schütte C, von Kleist M.Pharmacokinetics and pharmacodynamics of the reverse transcriptase inhibitor tenofovir and prophylactic efficacy against HIV-1 infection. PLoS One. 2012;7(7):e40382. doi: 10.1371/journal.pone.0040382. Epub 2012 Jul 11.
- Ergosterol peroxide
Catalog No.:BCN4897
CAS No.:2061-64-5
- Tetrahydromagnolol
Catalog No.:BCN8255
CAS No.:20601-85-8
- H-D-Asn-OH.H2O
Catalog No.:BCC2879
CAS No.:2058-58-4
- Oxytetracycline hydrochloride
Catalog No.:BCC9110
CAS No.:2058-46-0
- Calycosin
Catalog No.:BCN5930
CAS No.:20575-57-9
- SB273005
Catalog No.:BCC6501
CAS No.:205678-31-5
- Orexin B (human)
Catalog No.:BCC5765
CAS No.:205640-91-1
- Orexin A (human, rat, mouse)
Catalog No.:BCC5764
CAS No.:205640-90-0
- alpha-Chaconine
Catalog No.:BCN2162
CAS No.:20562-03-2
- alpha-Solanine
Catalog No.:BCN2701
CAS No.:20562-02-1
- Oxibendazole
Catalog No.:BCC4818
CAS No.:20559-55-1
- Pterocarpadiol D
Catalog No.:BCN7760
CAS No.:2055882-23-8
- CB30865
Catalog No.:BCC1457
CAS No.:206275-15-2
- Encecalin
Catalog No.:BCN4898
CAS No.:20628-09-5
- Calycosin-7-O-beta-D-glucoside
Catalog No.:BCN5931
CAS No.:20633-67-4
- Monomelittoside
Catalog No.:BCN8509
CAS No.:20633-72-1
- L-R4W2
Catalog No.:BCC5779
CAS No.:206350-79-0
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- Coniferaldehyde
Catalog No.:BCN4899
CAS No.:20649-42-7
- Sinapaldehyde
Catalog No.:BCN4900
CAS No.:20649-43-8
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
- Carmichaenine C
Catalog No.:BCN7731
CAS No.:2065228-61-5
- Carmichaenine D
Catalog No.:BCN7732
CAS No.:2065228-62-6


