Conantokin GNR2B-selective NMDA receptor antagonist CAS# 93438-65-4 |
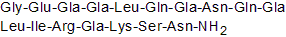
- AG-18
Catalog No.:BCC1051
CAS No.:118409-57-7
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- AZD8931 (Sapitinib)
Catalog No.:BCC3734
CAS No.:848942-61-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure
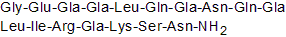
3D structure
| Cas No. | 93438-65-4 | SDF | Download SDF |
| PubChem ID | 16132376 | Appearance | Powder |
| Formula | C88H138N26O44 | M.Wt | 2264.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | GEXXLQXNQXLIRXKSN (Modifications: X = Gla, Asn-17 = C-terminal amide) | ||
| SMILES | CCC(C)C(C(=O)NC(CCCNC(=N)N)C(=O)NC(CC(C(=O)O)C(=O)O)C(=O)NC(CCCCN)C(=O)NC(CO)C(=O)NC(CC(=O)N)C(=O)N)NC(=O)C(CC(C)C)NC(=O)C(CC(C(=O)O)C(=O)O)NC(=O)C(CCC(=O)N)NC(=O)C(CC(=O)N)NC(=O)C(CC(C(=O)O)C(=O)O)NC(=O)C(CCC(=O)N)NC(=O)C(CC(C)C)NC(=O)C(CC(C(=O)O)C(=O)O)NC(=O)C(CC(C(=O)O)C(=O)O)NC(=O)C(CCC(=O)O)NC(=O)CN | ||
| Standard InChIKey | HTBKFGWATIYCSF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C88H138N26O44/c1-7-34(6)61(77(138)103-41(12-10-20-98-88(96)97)63(124)107-48(23-35(78(139)140)79(141)142)69(130)100-40(11-8-9-19-89)64(125)113-54(31-115)76(137)104-45(62(95)123)28-57(93)118)114-75(136)47(22-33(4)5)106-70(131)49(24-36(80(143)144)81(145)146)109-67(128)44(14-17-56(92)117)102-74(135)53(29-58(94)119)112-73(134)51(26-38(84(151)152)85(153)154)110-66(127)43(13-16-55(91)116)101-68(129)46(21-32(2)3)105-71(132)52(27-39(86(155)156)87(157)158)111-72(133)50(25-37(82(147)148)83(149)150)108-65(126)42(15-18-60(121)122)99-59(120)30-90/h32-54,61,115H,7-31,89-90H2,1-6H3,(H2,91,116)(H2,92,117)(H2,93,118)(H2,94,119)(H2,95,123)(H,99,120)(H,100,130)(H,101,129)(H,102,135)(H,103,138)(H,104,137)(H,105,132)(H,106,131)(H,107,124)(H,108,126)(H,109,128)(H,110,127)(H,111,133)(H,112,134)(H,113,125)(H,114,136)(H,121,122)(H,139,140)(H,141,142)(H,143,144)(H,145,146)(H,147,148)(H,149,150)(H,151,152)(H,153,154)(H,155,156)(H,157,158)(H4,96,97,98) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GluN2B (formally NR2B) selective, competitive antagonist of the NMDA receptor. Blocks NMDA-evoked current in mouse cortical neurons (IC50 = 480 nM); also inhibits NMDA-evoked responses in oocytes expressing GluN2B (formally NR2B), but not GluN2A (formally NR2A), subunits (IC50 ~300 nM). Exhibits neuroprotective properties in vivo and in vitro. |

Conantokin G Dilution Calculator

Conantokin G Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ENMD-2076
Catalog No.:BCC2186
CAS No.:934353-76-1
- HSP990 (NVP-HSP990)
Catalog No.:BCC5491
CAS No.:934343-74-5
- GSK1014802(CNV1014802)
Catalog No.:BCC6454
CAS No.:934240-30-9
- A-966492
Catalog No.:BCC2211
CAS No.:934162-61-5
- Venlafaxine
Catalog No.:BCC9190
CAS No.:93413-69-5
- Desvenlafaxine
Catalog No.:BCC5038
CAS No.:93413-62-8
- Chamaechromone
Catalog No.:BCN3718
CAS No.:93413-00-4
- 2-Benzothiazolol
Catalog No.:BCC8557
CAS No.:934-34-9
- 2-Aminobenzimidazole
Catalog No.:BCC8547
CAS No.:934-32-7
- 8beta-(2-Hydroxy-2-methyl-3-oxobutyryloxy)glucozaluzanin C
Catalog No.:BCN6682
CAS No.:93395-31-4
- 8-Epicrepiside E
Catalog No.:BCN7098
CAS No.:93395-30-3
- (S)-(-)-Atenolol
Catalog No.:BCC6633
CAS No.:93379-54-5
- Glucoliquiritin
Catalog No.:BCN6760
CAS No.:93446-18-5
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- 8-Deacetylyunaconitine
Catalog No.:BCN7609
CAS No.:93460-55-0
- Cobimetinib
Catalog No.:BCC1491
CAS No.:934660-93-2
- Cobimetinib (R-enantiomer)
Catalog No.:BCC1493
CAS No.:934660-94-3
- Cobimetinib (racemate)
Catalog No.:BCC1492
CAS No.:934662-91-6
- Karavilagenin D
Catalog No.:BCN4480
CAS No.:934739-29-4
- Glimepiride
Catalog No.:BCC2109
CAS No.:93479-97-1
- 2-Iodomelatonin
Catalog No.:BCC6772
CAS No.:93515-00-5
- (4->2)-Abeo-16-hydroxycleroda-2,13-dien-15,16-olide-3-al
Catalog No.:BCN7498
CAS No.:935293-70-2
- TH-237A
Catalog No.:BCC5378
CAS No.:935467-97-3
- Euphorbia factor L7a
Catalog No.:BCN3784
CAS No.:93550-94-8
Opposing action of conantokin-G on synaptically and extrasynaptically-activated NMDA receptors.[Pubmed:22306487]
Neuropharmacology. 2012 Jun;62(7):2227-38.
Synaptic and extrasynaptic activation of the N-methyl-D-aspartate receptor (NMDAR) has distinct consequences on cell signaling and neuronal survival. Since conantokin (con)-G antagonism is NR2B-selective, which is the key subunit involved in extrasynaptic activation of the receptor, its ability to specifically elicit distinct signaling outcomes in neurons with synaptically or extrasynaptically-activated NMDARs was evaluated. Inhibition of Ca(2+) influx through extrasynaptic NMDAR ion channels was neuroprotective, as it effectively enhanced levels of activated extracellular signal-regulated kinase 1/2 (ERK1/2), activated cAMP response element binding protein (CREB), enhanced mitochondrial viability, and attenuated the actin disorganization observed by extrasynaptic activation of NMDARs. Conversely, the pro-signaling pathways stimulated by synaptically-induced Ca(2+) influx were abolished by con-G. Furthermore, subunit non-selective con-T was unable to successfully redress the impairments in neurons caused by extrasynaptically-activated NMDARs, thus indicating that NR2B-specific antagonists are beneficial for neuron survival. Neurons ablated for the NR2B subunit showed weak synaptic Ca(2+) influx, reduced sensitivity to MK-801 blockage, and diminished extrasynaptic current compared to WT and NR2A(-/-) neurons. This indicates that the NR2B subunit is an integral component of both synaptic and extrasynaptic NMDAR channels. Altogether, these data suggest that con-G specifically targets the NR2B subunit in the synaptic and extrasynaptic locations, resulting in the opposing action of con-G on differentially activated pools of NMDARs.
Conantokin G-induced changes in the chemical coding of dorsal root ganglion neurons supplying the porcine urinary bladder.[Pubmed:22708364]
Pol J Vet Sci. 2012;15(1):101-9.
Conantokin G (CTG), isolated from the venom of the marine cone snail Conus geographus, is an antagonist of N-methyl-d-aspartate receptors (NMDARs), the activation of which, especially those located on the central afferent terminals and dorsal horn neurons, leads to hypersensitivity and pain. Thus, CTG blocking of NMDARs, has an antinociceptive effect, particularly in the case of neurogenic pain treatment. As many urinary bladder disorders are caused by hyperactivity of sensory bladder innervation, it seems useful to estimate the influence of CTG on the plasticity of sensory neurons supplying the organ. Retrograde tracer Fast Blue (FB) was injected into the urinary bladder wall of six juvenile female pigs. Three weeks later, intramural bladder injections of CTG (120 microg per animal) were carried out in all animals. After a week, dorsal root ganglia of interest were harvested from all animals and neurochemical characterization of FB+ neurons was performed using a routine double-immunofluorescence labeling technique on 10-microm-thick cryostat sections. CTG injections led to a significant decrease in the number of FB+ neurons containing substance P (SP), pituitary adenylate cyclase activating polypeptide (PACAP), somatostatin (SOM), calbindin (CB) and nitric oxide synthase (NOS) when compared with healthy animals (20% vs. 45%, 13% vs. 26%, 1.3% vs. 3%, 1.2 vs. 4% and 0.9% vs. 6% respectively) and to an increase in the number of cells immunolabelled for galanin (GAL, 39% vs. 6.5%). These data demonstrated that CTG changed the chemical coding of bladder sensory neurons, thus indicating that CTG could eventually be used in the therapy of selected neurogenic bladder illnesses.
Conantokin-G attenuates detrimental effects of NMDAR hyperactivity in an ischemic rat model of stroke.[Pubmed:25822337]
PLoS One. 2015 Mar 30;10(3):e0122840.
The neuroprotective activity of conantokin-G (con-G), a naturally occurring antagonist of N-methyl-D-aspartate receptors (NMDAR), was neurologically and histologically compared in the core and peri-infarct regions after ischemia/reperfusion brain injury in male Sprague-Dawley rats. The contralateral regions served as robust internal controls. Intrathecal injection of con-G, post-middle carotid artery occlusion (MCAO), caused a dramatic decrease in brain infarct size and swelling at 4 hr, compared to 26 hr, and significant recovery of neurological deficits was observed at 26 hr. Administration of con-G facilitated neuronal recovery in the peri-infarct regions as observed by decreased neurodegeneration and diminished calcium microdeposits at 4 hr and 26 hr. Intact Microtubule Associated Protein (MAP2) staining and neuronal cytoarchitecture was observed in the peri-infarct regions of con-G treated rats at both timepoints. Con-G restored localization of GluN1 and GluN2B subunits in the neuronal soma, but not that of GluN2A, which was perinuclear in the peri-infarct regions at 4 hr and 26 hr. This suggests that molecular targeting of the GluN2B subunit has potential for reducing detrimental consequences of ischemia. Overall, the data demonstrated that stroke-induced NMDAR excitoxicity is ameliorated by con-G-mediated repair of neurological and neuroarchitectural deficits, as well as by reconstituting neuronal localization of GluN1 and GluN2B subunits in the peri-infarct region of the stroked brain.
The selectivity of conantokin-G for ion channel inhibition of NR2B subunit-containing NMDA receptors is regulated by amino acid residues in the S2 region of NR2B.[Pubmed:19427876]
Neuropharmacology. 2009 Aug;57(2):127-36.
The conantokins are short, naturally occurring peptides that inhibit ion flow through N-methyl-d-aspartate receptor (NMDAR) channels. One member of this peptide family, conantokin-G (con-G), shows high selectivity for antagonism of NR2B-containing NMDAR channels, whereas other known conantokins are less selective inhibitors with regard to the nature of the NR2 subunit of the NMDAR complex. In order to define the molecular determinants of NR2B that govern con-G selectivity, we evaluated the ability of con-G to inhibit NMDAR ion channels expressed in human embryonic kidney (HEK)293 cells transfected with NR1, in combination with various NR2A/2B chimeras and point mutants, by electrophysiology using cells voltage-clamped in the whole-cell configuration. We found that a variant of the con-G-insensitive subunit, NR2A, in which the 158 residues comprising the S2 peptide segment (E(657)-I(814)) were replaced by the corresponding S2 region of NR2B (E(658)-I(815)), results in receptors that are highly sensitive to inhibition by con-G. Of the 22 amino acids that are different between the NR2A-S2 and the NR2B-S2 regions, exchange of one of these, M(739) of NR2B for the equivalent K(738) of NR2A, was sufficient to completely import the inhibitory activity of con-G into NR1b/NR2A-containing NMDARs. Some reinforcement of this effect was found by substitution of a second amino acid, K(755) of NR2B for Y(754) of NR2A. The discovery of the molecular determinants of NR2B selectivity with con-G has implications for the design of subunit-selective neurobiological probes and drug therapies, in addition to advancing our understanding of NR2B- versus NR2A-mediated neurological processes.
Selective antagonism of nigral neuropeptide responses to methamphetamine by conantokin G, a naturally occurring conopeptide.[Pubmed:10633160]
Eur J Pharmacol. 2000 Jan 3;387(1):55-8.
Some conopeptides derived from cone snails act on specific subunits of the NMDA receptor and thus, exert an influence on the dopamine system. In this study, one such conopeptide, Conantokin G, was administered i.c.v. in conjunction with methamphetamine, a potent central nervous system stimulant known to cause dopamine release and changes in tissue levels of neurotensin and dynorphin A in some brain structures. Both single and multiple administrations of the Conantokin G preferentially attenuated the methamphetamine-induced increases in tissue levels of these neuropeptides in the substantia nigra. Conantokin G also enhanced the behavioral effects of the methamphetamine.
Conantokin G is an NR2B-selective competitive antagonist of N-methyl-D-aspartate receptors.[Pubmed:10953056]
Mol Pharmacol. 2000 Sep;58(3):614-23.
Conantokin G (Con G) is a 17-amino-acid peptide antagonist of N-methyl-D-aspartate (NMDA) receptors isolated from the venom of the marine cone snail, Conus geographus. The mechanism of action of Con G has not been well defined; both competitive and noncompetitive interactions with the NMDA-binding site have been proposed. In this study the mechanism of action and subunit selectivity of Con G was examined in whole-cell voltage-clamp recordings from cultured neurons and in two electrode voltage-clamp recordings from Xenopus oocytes expressing recombinant NMDA receptors. Con G was a potent and selective antagonist of NMDA-evoked currents in murine cortical neurons (IC(50) = 480 nM). The slow onset of Con G block could be prevented by coapplication with high concentrations of NMDA or of the competitive antagonist (RS)-3-(2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid. Furthermore, in oocytes expressing NR1a/NR2B receptors, Con G produced a rightward shift in the concentration-response curve for NMDA, providing support for a competitive interaction with the NMDA-binding site. Con G produced an apparent noncompetitive shift in the concentration-response curve for spermine potentiation of NMDA responses, but this was due to spermine-induced enhancement of Con G block. Spermine produced a similar enhancement of DL-2-amino-S-phosphopentanoic acid block. Finally, Con G selectively blocked NMDA receptors containing the NR2B subunit. These results demonstrate that Con G is a subunit-specific competitive antagonist of NMDA receptors. The unique subunit selectivity profile of Con G may explain its favorable in vivo profile compared with nonselective NMDA antagonists.
Neuroprotective efficacy and therapeutic window of the high-affinity N-methyl-D-aspartate antagonist conantokin-G: in vitro (primary cerebellar neurons) and in vivo (rat model of transient focal brain ischemia) studies.[Pubmed:10871336]
J Pharmacol Exp Ther. 2000 Jul;294(1):378-86.
Conantokin-G (Con-G), a 17-amino-acid peptide derived from marine snails and a potent N-methyl-D-aspartate (NMDA) antagonist, was evaluated for its neuroprotective properties in vitro and in vivo. In primary cerebellar neurons, Con-G was shown to decrease excitotoxic calcium responses to NMDA and to exhibit differential neuroprotection potencies against hypoxia/hypoglycemia-, NMDA-, glutamate-, or veratridine-induced injury. Using the intraluminal filament method of middle cerebral artery occlusion as an in vivo rat model of transient focal brain ischemia, the neuroprotective dose-response effect of Con-G administration beginning 30 min postocclusion was evaluated after 2 h of ischemia and 22 h of reperfusion. In the core region of injury, an 89% reduction in brain infarction was measured with significant neurological and electroencephalographic recovery at the maximal dose tested (2 nmol), although mild sedation was noted. Lower doses of Con-G (0.001-0.5 nmol) were significantly neuroprotective without causing sedation. Postinjury time course experiments demonstrated a therapeutic window out to at least 4 to 8 h from the start of the injury, providing a 47% reduction in core injury. The neuroprotective effect of Con-G (0. 5 nmol) was also evaluated after 72 h of injury, where a 54% reduction in core brain infarction was measured. Critically, in both recovery models (i.e., 24 and 72 h), the reduction in brain infarction was associated with significant improvements in neurological and electroencephalographic recovery. These data provide evidence for the potent and highly efficacious effect of Con-G as a neuroprotective agent, with an excellent therapeutic window for the potential intervention against ischemic/excitotoxic brain injury.


