Gefitinib hydrochloridePotent EGFR inhibitor CAS# 184475-55-6 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 184475-55-6 | SDF | Download SDF |
| PubChem ID | 19077490 | Appearance | Powder |
| Formula | C22H25Cl2FN4O3 | M.Wt | 483.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ZD-1839 hydrochloride | ||
| Solubility | H2O : 6.25 mg/mL (12.93 mM; Need ultrasonic) DMSO : 0.227 mg/mL (0.47 mM; Need ultrasonic and warming) | ||
| Chemical Name | N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine;hydrochloride | ||
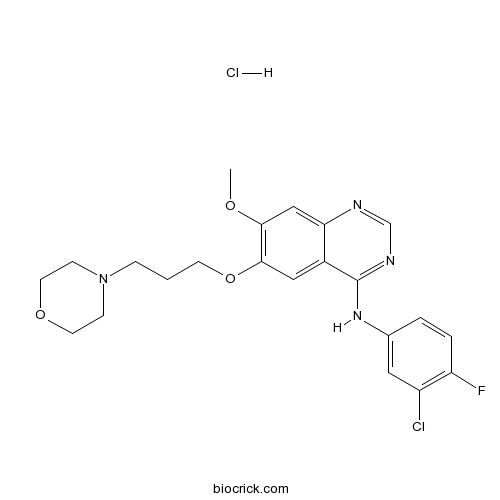
| SMILES | COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4.Cl | ||
| Standard InChIKey | QUINXWLATMJDQF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H24ClFN4O3.ClH/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15;/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gefitinib hydrochloride is an inhibitor that specifically binds and inhibits the EGFR tyrosine kinase, with the IC50 value of 2-37 nM in NR6wtEGFR cells.In Vitro:Gefitinib (0.01-0.1 mM) results in increased phosphotyrosine load of the receptor, increased signalling to ERK and stimulation of proliferation and anchorage-independent growth, presumably by inducing EGFRvIII dimerisation in long-term exposure of EGFRvIII-expressing cells. On the other hand, gefitinib (1-2 mM) significantly decreases EGFRvIII phosphotyrosine load, EGFRvIII-mediated proliferation and anchorage-independent growth[1]. Gefitinib (ZD1839) inhibits the monolayer growth of these EGF-driven untransformed cells with IC50 of 20 nM[2]. Gefitinib leads to an inhibition of CALU-3 and GLC82 cell proliferation, with an IC50 of 2 μM[3].In Vivo:Gefitinib (150 mg/kg, p.o.) in conbination with Metformin induces a significant reduction in tumor growth in nude mice bearing H1299 or CALU-3 GEF-R cells that are grown subcutaneously as tumor xenografts[3]. In irradiated rats, Gefitinib treatment augmentes lung inflammation, including inflammatory cell infiltration and pro-inflammatory cytokine expression, while Gefitinib treatment attenuates fibrotic lung remodeling due to the inhibition of lung fibroblast proliferation[4]. References: | |||||

Gefitinib hydrochloride Dilution Calculator

Gefitinib hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0689 mL | 10.3443 mL | 20.6885 mL | 41.377 mL | 51.7213 mL |
| 5 mM | 0.4138 mL | 2.0689 mL | 4.1377 mL | 8.2754 mL | 10.3443 mL |
| 10 mM | 0.2069 mL | 1.0344 mL | 2.0689 mL | 4.1377 mL | 5.1721 mL |
| 50 mM | 0.0414 mL | 0.2069 mL | 0.4138 mL | 0.8275 mL | 1.0344 mL |
| 100 mM | 0.0207 mL | 0.1034 mL | 0.2069 mL | 0.4138 mL | 0.5172 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
The EGFR is a Mr 170,000 transmembrane glycoprotein with an external binding domain and an intracellular tyrosine kinase domain. Gefitinib (ZD-1839, Iressa) is an Epidermal Growth Factor Receptor-selective Tyrosine Kinase Inhibitor.
In vitro: Gefitinib inhibited colony formation in soft agar in a dose dependent manner in all cancer cell lines. However, treatment with higher doses resulted in a 2–4-fold increases in apoptosis. Dose-dependent supra-additive increase in growth inhibition was observed when cancer cells were treated with totoxic drugs and Gefitinib. The combined treatment markedly enhanced apoptotic cell death induced by single agent treatment [1].
In vivo: Gefitinib treatment of nude mice bearing established human GEO colon cancer xenografts revealed a reversible dose-dependent inhibition of tumor growth because GEO tumors resumed the growth rate of controls at the end of the treatment [1].
Clinical trial: Administration of a 250-mg dose of gefitinib as a dispersion preparation by drink or nasogastric tube achieved a systemic exposure to gefitinib that was consistent with that achieved when gefitinib was administered as a whole tablet. No evidence of tolerability problems associated with the routes of administration studied was observed in these healthy volunteers [2].
References:
[1] Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6(5):2053-63.
[2] Cantarini MV, McFarquhar T, Smith RP, Bailey C, Marshall AL. Relative bioavailability and safety profile of gefitinib administered as a tablet or as a dispersion preparation via drink or nasogastric tube: results of a randomized, open-label, three-period crossover study in healthy volunteers. Clin Ther. 2004;26(10):1630-6.
- Gefitinib
Catalog No.:BCN2173
CAS No.:184475-35-2
- Cucurbitacin E
Catalog No.:BCN2300
CAS No.:18444-66-1
- Vitisin B
Catalog No.:BCN6697
CAS No.:142449-90-9
- Picfeltarraenin IV
Catalog No.:BCN2852
CAS No.:184288-35-5
- Dihydromorin
Catalog No.:BCN1149
CAS No.:18422-83-8
- SR 142948
Catalog No.:BCC7323
CAS No.:184162-64-9
- GB 2a
Catalog No.:BCN7425
CAS No.:18412-96-9
- Hautriwaic acid
Catalog No.:BCN4686
CAS No.:18411-75-1
- Isoleojaponin
Catalog No.:BCN7442
CAS No.:1840966-49-5
- Calystegine B4
Catalog No.:BCN1881
CAS No.:184046-85-3
- Dimeric coniferyl acetate
Catalog No.:BCN1148
CAS No.:184046-40-0
- sitaxsentan
Catalog No.:BCC1951
CAS No.:184036-34-8
- Madecassic acid
Catalog No.:BCN1013
CAS No.:18449-41-7
- Bakkenolide B
Catalog No.:BCN7207
CAS No.:18455-98-6
- 1-Oxobakkenolide S
Catalog No.:BCN7114
CAS No.:18456-02-5
- Bakkenolide D
Catalog No.:BCN2909
CAS No.:18456-03-6
- Taxinine B
Catalog No.:BCN1150
CAS No.:18457-44-8
- 7-Deacetoxytaxinine J
Catalog No.:BCN7677
CAS No.:18457-45-9
- Taxinine J
Catalog No.:BCN6943
CAS No.:18457-46-0
- ROS 234 dioxalate
Catalog No.:BCC7245
CAS No.:184576-87-2
- Mangostanol
Catalog No.:BCN1151
CAS No.:184587-72-2
- Ethyl Coumarin-3-Carboxylate
Catalog No.:BCC9228
CAS No.:1846-76-0
- Nigracin
Catalog No.:BCN1152
CAS No.:18463-25-7
- 2-C-Methyl-D-erythrono-1,4-lactone
Catalog No.:BCN4769
CAS No.:18465-71-9
Phosphatase and tensin homolog deleted on chromosome 10 degradation induced by NEDD4 promotes acquired erlotinib resistance in non-small-cell lung cancer.[Pubmed:28714370]
Tumour Biol. 2017 Jul;39(7):1010428317709639.
Acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors, such as gefitinib and erlotinib, is a critical issue in the treatment of patients with epidermal growth factor receptor mutant-positive non-small-cell lung cancer. Recent evidence suggests that downregulation of gene of phosphatase and tensin homolog deleted on chromosome 10 plays an important role in acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors in various types of cancers, including lung cancer. It was reported that the E3 ubiquitin ligase neural precursor cell expressed developmentally downregulated gene (NEDD4) (also known as NEDD4-1) negatively regulated phosphatase and tensin homolog deleted on chromosome 10 protein levels through poly-ubiquitination and proteolysis in carcinomas of the prostate, lung, and bladder. Whether this process plays a role in epidermal growth factor receptor-tyrosine kinase inhibitors resistance in non-small-cell lung cancer has not been studied extensively. In view of this, we investigated the involvement of NEDD4 and phosphatase and tensin homolog deleted on chromosome 10 in acquired erlotinib resistance with tyrosine kinase inhibitor-sensitive (HCC827) or tyrosine kinase inhibitor-resistant (Erlotinib-resistant HCC827/ER cells which harbored exon 19 deletion. Overexpression of NEDD4 in HCC827/ER cells was detected, and the reverse correlation between NEDD4 and phosphatase and tensin homolog deleted on chromosome 10 expression in these cells was also revealed. In HCC827/ER cells with knockdown of NEDD4, phosphatase and tensin homolog deleted on chromosome 10 and p-Akt expressions were decreased; the sensitivity of HCC827/ER cells to erlotinib was partially restored. Similar results were also observed in vivo. In H1650/ER cells harboring both exon 19 and phosphatase and tensin homolog deleted on chromosome 10 deletion, expression of p-Akt and sensitivity to erlotinib were not affected by simple knockdown of NEDD4 but affected after transfection of phosphatase and tensin homolog deleted on chromosome 10 into H1650/ER cells. Our results demonstrate that NEDD4 may promote the acquired resistance of non-small-cell lung cancer cells to erlotinib by decreasing phosphatase and tensin homolog deleted on chromosome 10 protein expression. Targeted decrease in NEDD4 expression may be a potential therapeutic strategy for tyrosine kinase inhibitor-resistant non-small-cell lung cancer.
Cytochrome P450 3A selectively affects the pharmacokinetic interaction between erlotinib and docetaxel in rats.[Pubmed:28716728]
Biochem Pharmacol. 2017 Nov 1;143:129-139.
Erlotinib as a first-line drug is used in non-small cell lung cancer (NSCLC) patients with sensitive EGFR mutations, while resistance to this drug will occur after several years of treatment. Therefore, the microtubule disturber docetaxel is introduced as combined regimen in clinical trials. This report investigated the potentials and mechanisms of drug-drug interaction (DDI) between erlotinib and docetaxel using wild type (WT) and Cyp3a1/2 knockout (KO) rats. The erlotinib O-demethylation and docetaxel hydroxylation reactions in the absence or the presence of another drug were analyzed in vitro via the assay of rat liver microsomes. In whole animal studies, erlotinib and docetaxel were given to WT and KO rats individually or jointly, and the pharmacokinetic profiles of these two drugs were analyzed and compared among different groups. The results showed that docetaxel not only inhibited the CYP3A-mediated biotransformation of erlotinib in vitro, but also significantly increased the maximum concentration and systemic exposure of erlotinib in vivo in WT rats. In contrast, the DDI was significantly attenuated in KO rats. On the other hand, erlotinib did not influence docetaxel either in vitro biotransformation or in vivo pharmacokinetic behaviors. These results exhibited the potentials of erlotinib-docetaxel interaction and indicated that the CYP3A played the perpetrating role of docetaxel on erlotinib in rats. A better understanding of this DDI with CYP3A may help the regulation of the use of these two drugs, avoid potential problems, and adjust dose carefully and early in clinic.
Effects of an Alkaline Diet on EGFR-TKI Therapy in EGFR Mutation-positive NSCLC.[Pubmed:28870946]
Anticancer Res. 2017 Sep;37(9):5141-5145.
BACKGROUND: The acidic tumor microenvironment is associated with progression of cancers. The purpose of this study was to investigate the association between an alkaline diet and the effect of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) in non-small cell lung cancer (NSCLC) patients. PATIENTS AND METHODS: Eleven advanced or recurrent NSCLC patients with EGFR mutations treated with EGFR-TKI after being instructed to follow an alkaline diet were retrospectively evaluated. RESULTS: The median progression-free survival (PFS) and overall survival (OS) were 19.5 (range=3.1-33.8) and 28.5 (range=15.4-46.6) months. The average dosage of EGFR-TKI was 56+/-22% of the standard dosage. Urine pH was significantly increased after the alkaline diet (6.00+/-0.38 vs. 6.95+/-0.55; p<0.05). CONCLUSION: An alkaline diet may enhance the effect of EGFR-TKI treatment in NSCLC patients with EGFR mutations.
Impact of Small Molecules on beta-Catenin and E-Cadherin Expression in HPV16-positive and -negative Squamous Cell Carcinomas.[Pubmed:28551620]
Anticancer Res. 2017 Jun;37(6):2845-2852.
BACKGROUND: The validation of potential molecular targets in head and neck squamous cell carcinoma (SCC) is mandatory. beta-Catenin and E-cadherin are crucial for cancer progression through epithelial-mesenchymal transition. We analyzed the effect of the tyrosine kinase inhibitors nilotinib, dasatinib, erlotinib and gefitinib on beta-catenin and E-cadherin expression in SCC with respect to human papillomavirus (HPV) status. MATERIALS AND METHODS: Expression of beta-catenin and E-cadherin in cell lines UMSCC 11A, UMSCC 14C and CERV196 under the influence of tyrosine kinase inhibitors were analyzed by enzyme-linked immunosorbent assay. RESULTS: All agents reduced beta-catenin and E-cadherin expression of HPV16-negative cells. Increased E-cadherin expression was observed after treatment with gefitinib and dasatinib in HPV16-positive cells. CONCLUSION: All substances, nilotinib, dasatinib, erlotinib and gefitinib have a significant impact on beta-catenin and E-cadherin expression in both HPV16-positive and HPV16-negative cells in vitro. Alterations of beta-catenin and E-cadherin could provide novel insights for future targeted therapies of head and neck SCC.
Treatment in EGFR-mutated non-small cell lung cancer: how to block the receptor and overcome resistance mechanisms.[Pubmed:28708233]
Tumori. 2017 Jul 31;103(4):325-337.
In non-small cell lung cancer (NSCLC), the identification of epidermal growth factor receptor (EGFR) mutations and the parallel development of EGFR tyrosine kinase inhibitors (TKIs) have radically changed the therapeutic management strategies. Currently, erlotinib, gefitinib, and afatinib are all approved as standard first-line treatment in EGFR-mutated NSCLC. However, despite the proven efficacy, some EGFR-mutated NSCLCs do not respond to EGFR TKIs, while some patients, after a favorable and prolonged response to EGFR TKIs, inevitably progress within about 10-14 months. Epidermal growth factor receptor-dependent mechanisms, activation of alternative pathways, or phenotypic transformation can cause the resistance to EGFR TKIs. The exon 20 p.Thr790Met point mutation (T790M) is responsible for about 60% of cases of resistance when progression occurs. A third-generation TKI, osimertinib, improved outcome in patients harboring T790M after first- and second-generation TKI treatment. However, resistance develops even after treatment with third-generation drugs. To date, the Cys797Ser (C797S) mutation in exon 20 of EGFR is the most well-known resistance mutation after osimertinib. Fourth-generation TKIs are already under development. Nevertheless, additional information is needed to better understand and effectively overcome resistance. The aim of this review is to report recent advances and future perspectives in the treatment of EGFR-mutated NSCLC, highlighting the resistance mechanisms that underlie disease progression.


