A-966492PARP-1/-2 inhibitor, highly potent CAS# 934162-61-5 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SD-208
Catalog No.:BCC1938
CAS No.:627536-09-8
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 934162-61-5 | SDF | Download SDF |
| PubChem ID | 16666333 | Appearance | Powder |
| Formula | C18H17FN4O | M.Wt | 324.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (308.31 mM) *"≥" means soluble, but saturation unknown. | ||
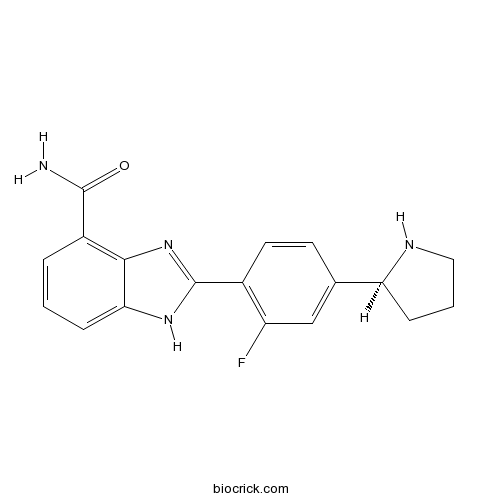
| Chemical Name | 2-[2-fluoro-4-[(2S)-pyrrolidin-2-yl]phenyl]-1H-benzimidazole-4-carboxamide | ||
| SMILES | C1CC(NC1)C2=CC(=C(C=C2)C3=NC4=C(C=CC=C4N3)C(=O)N)F | ||
| Standard InChIKey | AHIVQGOUBLVTCB-AWEZNQCLSA-N | ||
| Standard InChI | InChI=1S/C18H17FN4O/c19-13-9-10(14-5-2-8-21-14)6-7-11(13)18-22-15-4-1-3-12(17(20)24)16(15)23-18/h1,3-4,6-7,9,14,21H,2,5,8H2,(H2,20,24)(H,22,23)/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A-966492 is a novel and potent inhibitor of PARP1 and PARP2 with Ki of 1 nM and 1.5 nM, respectively. | |||||
| Targets | PARP1 | PARP2 | ||||
| IC50 | 1 nM | 1.5 nM | ||||

A-966492 Dilution Calculator

A-966492 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0831 mL | 15.4154 mL | 30.8309 mL | 61.6618 mL | 77.0772 mL |
| 5 mM | 0.6166 mL | 3.0831 mL | 6.1662 mL | 12.3324 mL | 15.4154 mL |
| 10 mM | 0.3083 mL | 1.5415 mL | 3.0831 mL | 6.1662 mL | 7.7077 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6166 mL | 1.2332 mL | 1.5415 mL |
| 100 mM | 0.0308 mL | 0.1542 mL | 0.3083 mL | 0.6166 mL | 0.7708 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A-966492 is an inhibitor of PARP-1 with Ki value of 1nM [1].
PARP-1 belongs to the poly(ADP-ribose) polymerases (PARPs) family, it contributes to the resistance happened after cancer therapy. A-966492 is a potent inhibitor of both PARP-1 and PARP-2 (Ki value of 1.5nM) with good potency in C41 whole cells (EC50 value of 1nM). A-966492 shows excellent pharmaceutical properties with oral bioavailabilities of 34-72% and half-lives of 1.7-1.9 h. Additionally, A-966492 can crosses the blood-brain barrier. A-966492 is proved potent in a murine B16F10 syngeneic melanoma model and a BRCA1-deficient MX-1 breast carcinoma model. Meanwhile, it can enhance the efficacy of TMZ and carboplatin in these models [1].
References:
[1] Penning TD, Zhu GD, Gong J, Thomas S, Gandhi VB, Liu X, Shi Y, Klinghofer V, Johnson EF, Park CH, Fry EH, Donawho CK, Frost DJ, Buchanan FG, Bukofzer GT, Rodriguez LE, Bontcheva-Diaz V, Bouska JJ, Osterling DJ, Olson AM, Marsh KC, Luo Y, Giranda VL. Optimization of phenyl-substituted benzimidazole carboxamide poly(ADP-ribose) polymerase inhibitors: identification of (S)-2-(2-fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benzimidazole-4-carboxamide (A-966492), a highly potent and efficacious inhibitor. J Med Chem. 2010 Apr 22;53(8):3142-53.
- Venlafaxine
Catalog No.:BCC9190
CAS No.:93413-69-5
- Desvenlafaxine
Catalog No.:BCC5038
CAS No.:93413-62-8
- Chamaechromone
Catalog No.:BCN3718
CAS No.:93413-00-4
- 2-Benzothiazolol
Catalog No.:BCC8557
CAS No.:934-34-9
- 2-Aminobenzimidazole
Catalog No.:BCC8547
CAS No.:934-32-7
- 8beta-(2-Hydroxy-2-methyl-3-oxobutyryloxy)glucozaluzanin C
Catalog No.:BCN6682
CAS No.:93395-31-4
- 8-Epicrepiside E
Catalog No.:BCN7098
CAS No.:93395-30-3
- (S)-(-)-Atenolol
Catalog No.:BCC6633
CAS No.:93379-54-5
- (+)-Taddol
Catalog No.:BCC8378
CAS No.:93379-49-8
- 20-Hydroxy-3-oxo-28-lupanoic acid
Catalog No.:BCN4478
CAS No.:93372-87-3
- Monomethyl lithospermate
Catalog No.:BCN8124
CAS No.:933054-33-2
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
- GSK1014802(CNV1014802)
Catalog No.:BCC6454
CAS No.:934240-30-9
- HSP990 (NVP-HSP990)
Catalog No.:BCC5491
CAS No.:934343-74-5
- ENMD-2076
Catalog No.:BCC2186
CAS No.:934353-76-1
- Conantokin G
Catalog No.:BCC6120
CAS No.:93438-65-4
- Glucoliquiritin
Catalog No.:BCN6760
CAS No.:93446-18-5
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- 8-Deacetylyunaconitine
Catalog No.:BCN7609
CAS No.:93460-55-0
- Cobimetinib
Catalog No.:BCC1491
CAS No.:934660-93-2
- Cobimetinib (R-enantiomer)
Catalog No.:BCC1493
CAS No.:934660-94-3
- Cobimetinib (racemate)
Catalog No.:BCC1492
CAS No.:934662-91-6
- Karavilagenin D
Catalog No.:BCN4480
CAS No.:934739-29-4
- Glimepiride
Catalog No.:BCC2109
CAS No.:93479-97-1
Optimization of phenyl-substituted benzimidazole carboxamide poly(ADP-ribose) polymerase inhibitors: identification of (S)-2-(2-fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benzimidazole-4-carboxamide (A-966492), a highly potent and efficacious inhibitor.[Pubmed:20337371]
J Med Chem. 2010 Apr 22;53(8):3142-53.
We have developed a series of phenylpyrrolidine- and phenylpiperidine-substituted benzimidazole carboxamide poly(ADP-ribose) polymerase (PARP) inhibitors with excellent PARP enzyme potency as well as single-digit nanomolar cellular potency. These efforts led to the identification of (S)-2-(2-fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benzimidazole-4-carboxamide (22b, A-966492). Compound 22b displayed excellent potency against the PARP-1 enzyme with a K(i) of 1 nM and an EC(50) of 1 nM in a whole cell assay. In addition, 22b is orally bioavailable across multiple species, crosses the blood-brain barrier, and appears to distribute into tumor tissue. It also demonstrated good in vivo efficacy in a B16F10 subcutaneous murine melanoma model in combination with temozolomide and in an MX-1 breast cancer xenograft model both as a single agent and in combination with carboplatin.
The combination of A-966492 and Topotecan for effective radiosensitization on glioblastoma spheroids.[Pubmed:28797568]
Biochem Biophys Res Commun. 2017 Sep 30;491(4):1092-1097.
Radiotherapy is one of the modalities in the treatment of glioblastoma patients, but glioma tumors are resistant to radiation and also chemotherapy drugs. Thus, researchers are investigating drugs which have radiosensitization capabilities in order to improve radiotherapy. PARP enzymes and topoisomerase I enzymes have a critical role in repairing DNA damage in tumor cells. Thus, inhibiting activity of these enzymes helps stop DNA damage repair and increase DSB lethal damages. In the current study, we investigated the combination of TPT as a topoisomerase I inhibitor, and A-966492 as a novel PARP inhibitor for further radiosensitization. U87MG cells (a human glioblastoma cell line) were cultured in Poly-Hema coated flasks to reach 300 mum-diameter spheroids. Treatments were accomplished by using non-toxic concentrations of A-966492 and Topotecan. The surviving fraction of treated cells was determined by clonogenic assay after treatment with drugs and 6 MV X-ray. The gamma-H2AX expression was measured by an immunofluorescence staining method to examine the influence of A-966492, TPT and radiation on the induction of double stranded DNA breaks. Treatments using the A-966492 drug were conducted in concentration of 1 muM. Combining A-966492 and TPT with radiation yielded enhanced cell killing, as demonstrated by a sensitizer enhancement ratio at 50% survival (SER50) 1.39 and 1.16 respectively. Radio- and chemo-sensitization was further enhanced when A-966492 was combined with both X-ray and TPT, with SER50 of 1.53. Also gamma-H2AX expression was higher in the group treated with a combination of drugs and radiation. A-966492 is an effective PARP inhibitor and has significant radio-sensitivity on U87MG spheroids. By accumulating cells in the S phase and by inhibiting the DNA damage repair, TPT enhanced radio-sensitivity. A-966492 combined with TPT as a topoisomerase I inhibitor had additive radio-sensitizing effects. As a result, applying PARP and topoisomerase I inhibitors can be a suitable strategy for improving radiotherapy in clinics.


