BavisantHuman H3 receptor antagonist CAS# 929622-08-2 |
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 929622-08-2 | SDF | Download SDF |
| PubChem ID | 16061509 | Appearance | Powder |
| Formula | C19H27N3O2 | M.Wt | 329.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
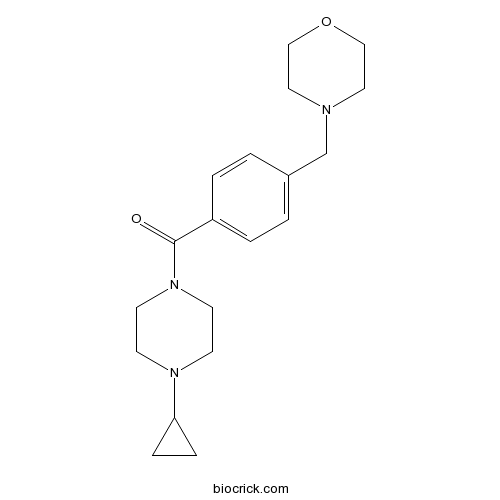
| Chemical Name | (4-cyclopropylpiperazin-1-yl)-[4-(morpholin-4-ylmethyl)phenyl]methanone | ||
| SMILES | C1CC1N2CCN(CC2)C(=O)C3=CC=C(C=C3)CN4CCOCC4 | ||
| Standard InChIKey | BGBVSGSIXIIREO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H27N3O2/c23-19(22-9-7-21(8-10-22)18-5-6-18)17-3-1-16(2-4-17)15-20-11-13-24-14-12-20/h1-4,18H,5-15H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bavisant Dilution Calculator

Bavisant Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0355 mL | 15.1773 mL | 30.3545 mL | 60.7091 mL | 75.8864 mL |
| 5 mM | 0.6071 mL | 3.0355 mL | 6.0709 mL | 12.1418 mL | 15.1773 mL |
| 10 mM | 0.3035 mL | 1.5177 mL | 3.0355 mL | 6.0709 mL | 7.5886 mL |
| 50 mM | 0.0607 mL | 0.3035 mL | 0.6071 mL | 1.2142 mL | 1.5177 mL |
| 100 mM | 0.0304 mL | 0.1518 mL | 0.3035 mL | 0.6071 mL | 0.7589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Bavisant (JNJ-31001074) is a highly selective, orally active antagonist of the human H3 receptor with a novel mechanism of action, involving wakefulness and cognition, with potential as a treatment for ADHD. Bavisant completed a phase II ADHD trial, but no results have been reported [1]. in vitro: N/A in vivo: Mean change from baseline in the total ADHD-RS-IV score at day 42 (primary efficacy endpoint) was -8.8 in the placebo group versus -9.3, -11.2 and -12.2 in the bavisant 1?mg/day, 3?mg/day and 10?mg/day groups, respectively; the change in the 10?mg/day group was not statistically superior to placebo (p=0.161), and hence statistical comparisons of the 1?mg/day and 3?mg/day groups with placebo based on a step-down closed testing procedure were not performed [2]. Clinical trial: A Study to Characterize the Pharmacokinetics and Effect of Food on JNJ-31001074 in Healthy Volunteers. Phase 2
- Fmoc-Tyr-OH
Catalog No.:BCC3562
CAS No.:92954-90-0
- LUF 6283
Catalog No.:BCC6318
CAS No.:92933-48-7
- 7-Oxo-ganoderic acid Z
Catalog No.:BCN7973
CAS No.:929248-72-6
- GSK461364
Catalog No.:BCC3788
CAS No.:929095-18-1
- Pracinostat (SB939)
Catalog No.:BCC2152
CAS No.:929016-96-6
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
- PF-03716556
Catalog No.:BCC2084
CAS No.:928774-43-0
- IRAK inhibitor 2
Catalog No.:BCC1655
CAS No.:928333-30-6
- AS 1892802
Catalog No.:BCC6335
CAS No.:928320-12-1
- MN 64
Catalog No.:BCC6489
CAS No.:92831-11-3
- Boc-D-Asp-OBzl
Catalog No.:BCC3370
CAS No.:92828-64-3
- 3-Chloro-1-(4-octylphenyl)-propanone
Catalog No.:BCN2249
CAS No.:928165-59-7
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
- Sessilifoline A
Catalog No.:BCN4473
CAS No.:929637-35-4
- Cucumegastigmane I
Catalog No.:BCN4474
CAS No.:929881-46-9
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
- 3,4-Dimethoxybenzyl Alcohol
Catalog No.:BCN2721
CAS No.:93-03-8
- 3,4-Dimethoxybenzoic acid
Catalog No.:BCN4475
CAS No.:93-07-2
- 2-Acetonaphthone
Catalog No.:BCC8510
CAS No.:93-08-3
- Guaifenesin
Catalog No.:BCN2977
CAS No.:93-14-1
- Methyleugenol
Catalog No.:BCN4074
CAS No.:93-15-2
- Methyl isoeugenol
Catalog No.:BCN8462
CAS No.:93-16-3
- N-(2-Methoxyphenyl)acetamide
Catalog No.:BCC9054
CAS No.:93-26-5
- Acetylisoeugenol
Catalog No.:BCN7075
CAS No.:93-29-8
Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder.[Pubmed:22519922]
CNS Drugs. 2012 May 1;26(5):421-34.
BACKGROUND: Psychostimulants, including methylphenidate and amphetamine preparations, are commonly prescribed for the treatment of attention-deficit hyperactivity disorder (ADHD) in children and adults. Histamine H3 receptors reside on non-histamine neurons and regulate other neurotransmitters (e.g. acetylcholine, noradrenaline [norepinephrine]) suggesting that H3 antagonists have the potential to improve attention and impulsivity. Research indicates that H3 receptor antagonists due to their novel mechanism of action may have a unique treatment effect offering an important alternative for the treatment of ADHD. Bavisant (JNJ-31001074) is a highly selective, orally active antagonist of the human H3 receptor with a novel mechanism of action, involving wakefulness and cognition, with potential as a treatment for ADHD. OBJECTIVE: The objective of this study was to evaluate the efficacy, safety and tolerability of three dosages of Bavisant compared with placebo in adults with ADHD. STUDY DESIGN: This randomized, double-blind, placebo- and active-controlled, parallel-group, multicentre study evaluated three dosages of Bavisant (1 mg/day, 3 mg/day or 10 mg/day) and two active controls in adults with ADHD. The study consisted of a screening phase of up to 14 days, a 42-day double-blind treatment phase and a 7-day post-treatment follow-up phase. Efficacy and safety assessments were performed. SETTING: The study was conducted at 37 study centres in the US from April 2009 through January 2010. PARTICIPANTS: Men and women aged 18-55 years with an established diagnosis of ADHD as confirmed by clinician and self-report diagnostic measures were enrolled. INTERVENTION: Participants were randomly assigned equally to one of six treatment groups: placebo, Bavisant 1 mg/day, 3 mg/day or 10 mg/day, atomoxetine hydrochloride 80 mg/day or osmotic-release oral system (OROS) methylphenidate hydrochloride 54 mg/day. MAIN OUTCOME MEASURE: The primary efficacy endpoint was the change in the Attention Deficit Hyperactivity Disorder Rating Scale, Version IV (ADHD-RS-IV) total score from baseline (day 1) to the end of the treatment phase (day 42), and included all randomized participants who received one or more doses of study drug and had baseline and one or more post-baseline assessments (intent-to-treat [ITT] population). Safety assessments included treatment-emergent adverse events (TEAEs), laboratory tests and ECG readings. RESULTS: 430 participants were randomized, 424 received one or more doses of study medication and 335 (78%) of those randomized completed the study. Study participants had a mean age of 33.9 years and were predominantly White men. Mean treatment duration ranged from 31.4 to 38.8 days across groups. Mean change from baseline in the total ADHD-RS-IV score at day 42 (primary efficacy endpoint) was -8.8 in the placebo group versus -9.3, -11.2 and -12.2 in the Bavisant 1 mg/day, 3 mg/day and 10 mg/day groups, respectively; the change in the 10 mg/day group was not statistically superior to placebo (p=0.161), and hence statistical comparisons of the 1 mg/day and 3 mg/day groups with placebo based on a step-down closed testing procedure were not performed. Mean change from baseline in the total ADHD-RS-IV score at day 42 was superior to placebo in the atomoxetine (-15.3) and OROS methylphenidate (-15.7) groups (p<0.005). Secondary efficacy assessments demonstrated a similar pattern with a non-significant trend towards improvement in the Bavisant groups. The two lower dosages showed a good tolerability profile, but the higher dosage of Bavisant was less well tolerated, as evidenced by the incidence of total TEAEs (61.8%, 82.4%, 89.0%), and discontinuations due to TEAEs (4.4%, 7.4%, 19.2%) in the Bavisant 1 mg/day, 3 mg/day and 10 mg/day groups, respectively, compared with 58.9% and 2.7%, respectively on placebo. In the atomoxetine and OROS methylphenidate groups, the incidence of total TEAEs was 83.8% and 82.4% and discontinuations due to TEAEs was 10.8% and 8.8%, respectively. CONCLUSION: Bavisant, a highly selective, wakefulness-promoting H3 antagonist, did not display significant clinical effectiveness in the treatment of adults with ADHD. CLINICAL TRIAL REGISTRATION NUMBER: NCT00880217.


