LoratadinePeripheral HH1R antagonist CAS# 79794-75-5 |
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 79794-75-5 | SDF | Download SDF |
| PubChem ID | 3957 | Appearance | Powder |
| Formula | C22H23ClN2O2 | M.Wt | 382.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SCH 29851 | ||
| Solubility | DMSO : 50 mg/mL (130.59 mM; Need ultrasonic) | ||
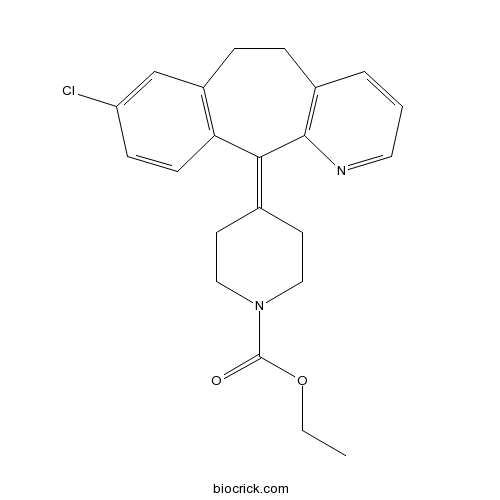
| Chemical Name | ethyl 4-(8-chloro-5,6-dihydrobenzo[1,2]cyclohepta[2,4-b]pyridin-11-ylidene)piperidine-1-carboxylate | ||
| SMILES | CCOC(=O)N1CCC(=C2C3=C(CCC4=C2N=CC=C4)C=C(C=C3)Cl)CC1 | ||
| Standard InChIKey | JCCNYMKQOSZNPW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peripheral histamine H1 receptor antagonist (Ki = 35 nM); devoid of central effects. Orally active antiallergic agent. |

Loratadine Dilution Calculator

Loratadine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6118 mL | 13.0589 mL | 26.1178 mL | 52.2357 mL | 65.2946 mL |
| 5 mM | 0.5224 mL | 2.6118 mL | 5.2236 mL | 10.4471 mL | 13.0589 mL |
| 10 mM | 0.2612 mL | 1.3059 mL | 2.6118 mL | 5.2236 mL | 6.5295 mL |
| 50 mM | 0.0522 mL | 0.2612 mL | 0.5224 mL | 1.0447 mL | 1.3059 mL |
| 100 mM | 0.0261 mL | 0.1306 mL | 0.2612 mL | 0.5224 mL | 0.6529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Peripheral histamine H1 receptor antagonist (Ki = 35 nM); devoid of central effects. Orally active antiallergic agent.
- H-Hyp(tBu)-OH
Catalog No.:BCC3249
CAS No.:79775-07-8
- Levonorgestrel
Catalog No.:BCC4792
CAS No.:797-63-7
- Linifanib (ABT-869)
Catalog No.:BCC1261
CAS No.:796967-16-3
- Crassicauline A
Catalog No.:BCN2516
CAS No.:79592-91-9
- Alarelin Acetate
Catalog No.:BCC1336
CAS No.:79561-22-1
- Sertraline HCl
Catalog No.:BCC5059
CAS No.:79559-97-0
- 1,7-Diphenyl-4-hepten-3-one
Catalog No.:BCN3592
CAS No.:79559-59-4
- 5-Ethoxychelerthrine
Catalog No.:BCC8105
CAS No.:79559-55-0
- L-165041
Catalog No.:BCC1687
CAS No.:79558-09-1
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
- Stelleranol
Catalog No.:BCN8014
CAS No.:795308-62-2
- Norketamine hydrochloride
Catalog No.:BCC5859
CAS No.:79499-59-5
- Castanospermine
Catalog No.:BCC6783
CAS No.:79831-76-8
- 1,2,3,6-Tetragalloylglucose
Catalog No.:BCN2159
CAS No.:79886-50-3
- Simvastatin
Catalog No.:BCN2569
CAS No.:79902-63-9
- Forsythoside A
Catalog No.:BCN1195
CAS No.:79916-77-1
- ML130 (Nodinitib-1)
Catalog No.:BCC4611
CAS No.:799264-47-4
- Idazoxan hydrochloride
Catalog No.:BCC6798
CAS No.:79944-56-2
- Boc-His(Bom)-OH
Catalog No.:BCC3400
CAS No.:79950-65-5
- Quinovic acid 3-O-beta-D-glucoside
Catalog No.:BCN4334
CAS No.:79955-41-2
- Fmoc-D-Ala-OH
Catalog No.:BCC3036
CAS No.:79990-15-1
- Blumeatin B
Catalog No.:BCN4335
CAS No.:79995-67-8
- Dapsone
Catalog No.:BCC5220
CAS No.:80-08-0
- Sulfamethoxypyridazine
Catalog No.:BCC4728
CAS No.:80-35-3
UV-Vis Spectrophotometry and Multivariate Calibration Method for Simultaneous Determination of Theophylline, Montelukast and Loratadine in Tablet Preparations and Spiked Human Plasma.[Pubmed:27980573]
Iran J Pharm Res. 2016 Summer;15(3):379-391.
Resolution of binary mixtures of theophylline (THEO), montelukast (MKST) and Loratadine (LORA) with minimum sample pre-treatment and without analyte separation has been successfully achieved by multivariate spectrophotometric calibration, together with partial least-squares (PLS-1), principal component regression (PCR) and hybrid linear analysis (HLA). Data of analysis were obtained from UV-Vis spectra of three compounds. The method of central composite design was used in the ranges of 2-14 and 3-11 mg L(-1) for calibration and validation sets, respectively. The models refinement procedure and their validation were performed by cross-validation. The minimum root mean square error of prediction (RMSEP) was 0.173 mg L(-1) for THEO with PCR, 0.187 mg L(-1) for MKST with PLS1 and 0.251 mg L(-1) for LORA with HLA techniques. The limit of detection was obtained 0.03, 0.05 and 0.05 mg L(-1) by PCR model for THEO, MKST and LORA, respectively. The procedure was successfully applied for simultaneous determination of the above compounds in pharmaceutical tablets and human plasma. Notwithstanding the spectral overlapping among three drugs, as well as the intrinsic variability of the latter in unknown samples, the recoveries are excellent.
Coamorphous Loratadine-Citric Acid System with Enhanced Physical Stability and Bioavailability.[Pubmed:28224393]
AAPS PharmSciTech. 2017 Oct;18(7):2541-2550.
Coamorphous systems using citric acid as a small molecular excipient were studied for improving physical stability and bioavailability of Loratadine, a BCS class II drug with low water solubility and high permeability. Coamorphous Loratadine-citric acid systems were prepared by solvent evaporation technique and characterized by differential scanning calorimetry, X-ray powder diffraction, and Fourier transform infrared spectroscopy. Solid-state analysis proofed that coamorphous Loratadine-citric acid system (1:1) was amorphous and homogeneous, had a higher T g over amorphous Loratadine, and the intermolecular hydrogen bond interactions between Loratadine and citric acid exist. The solubility and dissolution of coamorphous Loratadine-citric acid system (1:1) were found to be significantly greater than those of crystalline and amorphous form. The pharmacokinetic study in rats proved that coamorphous Loratadine-citric acid system (1:1) could significantly improve absorption and bioavailability of Loratadine. Coamorphous Loratadine-citric acid system (1:1) showed excellently physical stability over a period of 3 months at 25 degrees C under 0% RH and 25 degrees C under 60% RH conditions. The improved stability of coamorphous Loratadine-citric acid system (1:1) could be related to an elevated T g over amorphous form and the intermolecular hydrogen bond interactions between Loratadine and citric acid. These studies demonstrate that the developed coamorphous Loratadine-citric acid system might be a promising oral formulation for improving solubility and bioavailability of Loratadine.
When Hydromorphone Is Not Working, Try Loratadine: An Emergency Department Case of Loratadine as Abortive Therapy for Severe Pegfilgrastim-Induced Bone Pain.[Pubmed:27751704]
J Emerg Med. 2017 Feb;52(2):e29-e31.
BACKGROUND: Intractable bone pain is a notorious adverse effect of granulocyte-colony stimulating factors (G-CSFs), such as pegfilgrastim and filgrastim, which are given to help prevent neutropenia in patients who are undergoing chemotherapy. G-CSF-induced bone pain is surprisingly common and often refractory to treatment with conventional analgesics. CASE REPORT: This article describes an emergency department case of opiate and nonsteroidal anti-inflammatory drug-resistant pegfilgrastim-induced bone pain that was successfully alleviated with 10 mg of oral Loratadine, allowing for discharge home. WHY SHOULD AN EMERGENCY PHYSICIAN BE AWARE OF THIS?: This case suggests that Loratadine may be an easy to implement, safe, and effective therapy in the emergency department management of intractable bone pain caused by G-CSF use. Emergency physicians should be aware that Loratadine may successfully relieve otherwise intractable G-CSF-induced bone pain and allow for discharge home.
Development of novel electrochemical sensor on the base of molecular imprinted polymer decorated on SiC nanoparticles modified glassy carbon electrode for selective determination of loratadine.[Pubmed:27987666]
Mater Sci Eng C Mater Biol Appl. 2017 Feb 1;71:1106-1114.
A novel and selective electrochemical sensor was successfully developed for the determination of Loratadine by drop coating of synthesized Loratadine molecularly imprinted polymers on the surface of glassy carbon electrode modified with silicon carbide nanoparticles. The performance of the constructed sensor was evaluated by cyclic voltammetry, scanning electron microscopy, and electrochemical impedance spectroscopy techniques. Under optimized conditions, the resulting calibration curve exhibited a linear response within a concentration range of 1-33muM with a low detection limit (S/N=3) of 0.15muM. Finally, the proposed method was successfully applied as a selective, stabile, sensitive, simple, reproducibility electrochemical sensor with good repeatability for the determination of Loratadine in real samples and satisfactory results were obtained.
Antiallergic activity of loratadine, a non-sedating antihistamine.[Pubmed:2436504]
Allergy. 1987 Jan;42(1):57-63.
Loratadine is a new non-sedating antihistamine. The present studies compared Loratadine and terfenadine, another non-sedating antihistamine, for their ability to inhibit the bronchial response to histamine and other autacoids which have been implicated as contributing to the symptoms of an allergic reaction. In addition, the two antihistamines were evaluated in models of immunologically mediated allergic reactions. Loratadine is a more potent inhibitor of histamine-induced bronchospasm in guinea pigs than is terfenadine. Both antihistamines exhibit marked antiserotonin activity at doses 10 times their antihistamine ED50 values. In contrast, Loratadine and terfenadine produce little or no inhibition of the bronchial responses to methacholine, leukotriene C4 or platelet-activating factor. An allergic bronchospasm in guinea pigs is inhibited by Loratadine (ED50 = 0.40 mg/kg, p.o.) and terfenadine (ED50 = 1.7 mg/kg, p.o.). The bronchospasm associated with allergic anaphylaxis in rats is significantly inhibited by 10 mg/kg, p.o. Loratadine and 30 mg/kg, p.o. terfenadine. Loratadine exhibits antiallergy activity in vitro. At micromolar concentrations, Loratadine inhibits the release of histamine from Con A and A23187-stimulated rat peritoneal mast cells and the release of histamine and leukotriene C4 from a Con A-stimulated cloned murine mast cell line.
Selective displacement of [3H]mepyramine from peripheral vs. central nervous system receptors by loratadine, a non-sedating antihistamine.[Pubmed:2875889]
Eur J Pharmacol. 1986 Aug 7;127(1-2):153-5.
Displacement of [3H]mepyramine binding was compared in membranes from guinea-pig lung vs. cerebral cortex as a measure of affinity for peripheral vs. central nervous system (CNS) histamine receptors. Loratadine, a new non-sedating antihistamine, was found to be the only compound tested which was selective for lung (Ki = 35 nM) vs. cortex (Ki = 118 nM). This difference is statistically significant (P less than 0.05) whereas there was no significant (P greater than 0.05) difference in the Kis between the 2 tissues for terfenadine, astemizole, mequitazine or chlorpheniramine. It is concluded from these and other studies that the lack of significant sedative effects shown with Loratadine is due to its poor penetration into the CNS and selectivity for peripheral histamine receptors.
Evaluation of the CNS properties of SCH 29851, a potential non-sedating antihistamine.[Pubmed:6236679]
Agents Actions. 1984 Jun;14(5-6):590-7.
SCH 29851 [8-chloro[6,11-dihydro-11-(1-carboethoxy-4-piperidylidene)- 5-H-benzo [5,6]cyclohepta[1,2-b]-pyridine] was discovered as part of a search for a new antihistamine without effects on the central nervous system (CHS). Antihistaminic potency and duration of action of SCH 29851 and other antihistamines were assessed by inhibition of histamine-induced lethality in guinea pigs and histamine-induced paw edema in mice. Evaluation of possible CNS effects included gross observation of mice, rats, dogs and monkeys, prevention of electroshock-induced convulsions, acetic acid-induced writhing and physostigmine-induced lethality in mice and biochemical measures related to sedative liability such as displacement of in vivo 3H-mepyramine binding in mouse brain and in vitro 3H-WB 4101 binding in guinea pig cortex. Comparisons were made to several antihistamines considered to be sedative to varying degrees, including diphenhydramine, promethazine, chlorpheniramine and azatadine and to the newer antihistamines terfenadine and astemizole which are reported to be non-sedating in man at doses that antagonize the effects of histamine peripherally. SCH 29851 had antihistamine activity in the tests used with a potency at least comparable to most standards and was devoid of activity in all the functional and biochemical models used as indices of CNS activity. It is expected that SCH 29851 should be an effective, long acting, antihistamine in man without sedative effects at therapeutic doses.


