Mianserin HCl5-HT2 receptor antagonist CAS# 21535-47-7 |
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 21535-47-7 | SDF | Download SDF |
| PubChem ID | 68551 | Appearance | Powder |
| Formula | C18H21ClN2 | M.Wt | 300.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (166.21 mM; Need ultrasonic) | ||
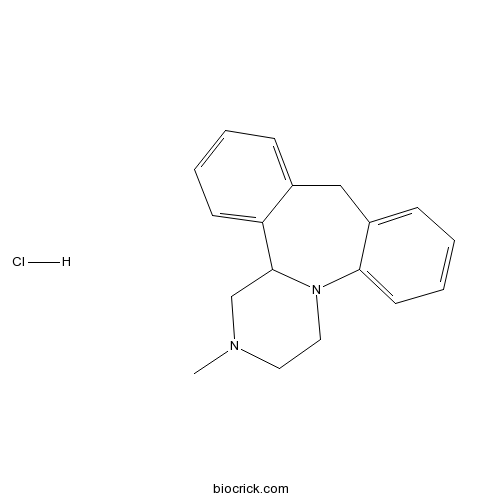
| Chemical Name | 1,2,3,4,10,14b-Hexahydro-2-methyldi | ||
| SMILES | [H+].[Cl-].CN1CCN2C(C1)c3ccccc3Cc4ccccc24 | ||
| Standard InChIKey | YNPFMWCWRVTGKJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-selective 5-HT2 receptor antagonist (Ki values are 0.0080 and 0.0081 μM for human 5-HT2A and 5-HT2C receptors expressed in HEK293 cells respectively). Has moderate affinity for 5-HT6. Antidepressant. |

Mianserin HCl Dilution Calculator

Mianserin HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3241 mL | 16.6207 mL | 33.2414 mL | 66.4827 mL | 83.1034 mL |
| 5 mM | 0.6648 mL | 3.3241 mL | 6.6483 mL | 13.2965 mL | 16.6207 mL |
| 10 mM | 0.3324 mL | 1.6621 mL | 3.3241 mL | 6.6483 mL | 8.3103 mL |
| 50 mM | 0.0665 mL | 0.3324 mL | 0.6648 mL | 1.3297 mL | 1.6621 mL |
| 100 mM | 0.0332 mL | 0.1662 mL | 0.3324 mL | 0.6648 mL | 0.831 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Non-selective 5-HT2 receptor antagonist. Has moderate affinity for 5-HT6. Antidepressant.
- Senampeline F
Catalog No.:BCN7804
CAS No.:71075-43-9
- BMS 753
Catalog No.:BCC6031
CAS No.:215307-86-1
- Betamethasone Valerate
Catalog No.:BCC3736
CAS No.:2152-44-5
- Pemoline
Catalog No.:BCC5967
CAS No.:2152-34-3
- BMS 493
Catalog No.:BCC7697
CAS No.:215030-90-3
- Methyl 2,6-dihydroxybenzoate
Catalog No.:BCN3563
CAS No.:2150-45-0
- Protocatechuic acid methyl ester
Catalog No.:BCN3542
CAS No.:2150-43-8
- 7,3',4'-Trihydroxyflavone
Catalog No.:BCN4674
CAS No.:2150-11-0
- Bruceine D
Catalog No.:BCN2894
CAS No.:21499-66-1
- Agrimonolide
Catalog No.:BCN4925
CAS No.:21499-24-1
- H-Arg(NO2)-OH
Catalog No.:BCC2864
CAS No.:2149-70-4
- 2-Deacetyltaxachitriene A
Catalog No.:BCN7415
CAS No.:214769-96-7
- SU 5402
Catalog No.:BCC1970
CAS No.:215543-92-3
- Sodium Dichloroacetate
Catalog No.:BCN2951
CAS No.:2156-56-1
- 23-deoxojessic acid
Catalog No.:BCN4926
CAS No.:215609-93-1
- Cyclocephaloside II
Catalog No.:BCC8310
CAS No.:215776-78-6
- SB269652
Catalog No.:BCC8052
CAS No.:215802-15-6
- SB-277011
Catalog No.:BCC1928
CAS No.:215803-78-4
- Bruceine E
Catalog No.:BCN7619
CAS No.:21586-90-3
- CX 546
Catalog No.:BCC7532
CAS No.:215923-54-9
- 7-Hydroxy-beta-carboline-1-propionic acid
Catalog No.:BCN1492
CAS No.:215934-15-9
- 15,16-Epoxy-12S-hydroxylabda-8(17),13(16),14-triene
Catalog No.:BCN1491
CAS No.:216011-55-1
- 1-Methyl-3-nitrophthalate
Catalog No.:BCC8468
CAS No.:21606-04-2
- β-Pompilidotoxin
Catalog No.:BCC1048
CAS No.:216064-36-7
Stereochemistry of serotonin receptor ligands from crystallographic data. Crystal structures of NAN-190.HBr, 1-phenylbiguanide, MDL 72222 and mianserin.HCl and selectivity criteria towards 5-HT1, 5-HT2, and 5-HT3 receptor subtypes.[Pubmed:8767764]
Acta Crystallogr B. 1996 Jun 1;52 ( Pt 3):509-18.
The crystal and molecular structures of the following serotoninergic drugs have been determined: (1) 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine hydrobromide hemihydrate (NAN-190.HBr), C23H28N3O3+.Br-.1/2H2O, M(r) = 483.42, monoclinic, C2/c, a = 21.916 (4), b = 15.207 (2), c = 14.052 (2) A, beta = 101.56 (1) degree, V = 4588 (1) A3, Z = 8, Dx = 1.40 Mgm-3, lambda (Mo K alpha) = 0.71069 A, mu = 1.823 mm-1, F(000) = 2008, T = 295 K, R = 0.035 for 2617 observed reflections; (2) N-phenylimidocarbonimidic diamide (1-phenylbiguanide), C8H11N5, M(r) = 177.21, monoclinic, P2(1)/c, a = 9.781 (2), b = 35.040(5), c = 11.000 (2) A, beta = 97.72(1) degree, V = 3736(1)A3, Z = 16, Dx = 1.26 Mg m-3, lambda (Mo K alpha) = 0.71069 A, mu = 0.084 mm-1, F(000) = 1504, T = 295 K, R = 0.070 for 3407 observed reflections; (3) 8-methyl-8-azabicyclo[3.2.1]oct-3yl 3,5-dicholorobenzoate (MDL 72222), C15H17Cl2NO2, M(r) = 314.21, triclinic, P1, alpha = 8.480 (3), b = 9.840 (3), c = 10.158 (4) A, alpha = 90.04 (3), beta = 111.77 (3), gamma = 105.07(3) degrees, V = 755.6(5) A3, Z = 2, Dx = 1.38 Mg m-3, lambda(Mo K alpha) = 0.71069 A, mu = 0.430 mm-1, F(000) = 328, T = 295 K, R = 0.070 for 1685 observed reflections; (4) 1, 2, 3, 4, 10, 14b-hexahydro-2-methyldibenzo[c.f]pyrizino[1, 2-alpha]azepine hydrochloride (mianserin. HCl), C18H21N2+. Cl-, M(r) = 300.83, monoclinic, P2(1)/a, a = 9.014 (2), b = 14.917 (2), c = 12.412 (2) A, beta = 108.84 (1) degree, V = 1579.5 (5) A3, Z = 4, Dx = 1.26 Mg m-3, lambda(Mo K alpha) = 0.71069 A, mu = 0.237 mm-1, F(000) = 640, T = 295 K, R = 0.063 for 1493 observed reflections. A systematic structural analysis of the present compounds and others known to interact with the 5-HT1, 5-HT2 and 5-HT3 receptors allows to identify their similarities with the endogenous ligand serotonin (5-HT) and the stereochemical differences which determine selectivity for the various receptor subtypes. The pharmacophoric feature for 5-HT receptor binding is identified in a constant-length vector linking an aromatic ring with a protonated nitrogen, while specific affinities for receptorial subtypes and the nature of the effect appear to be modulated by the dimensions of the substituents at nitrogen.
Interaction between the tetracyclic antidepressant mianserin HCl and opioid receptors.[Pubmed:9928920]
Eur Neuropsychopharmacol. 1998 Dec;8(4):297-302.
The antinociceptive effects of the tetracyclic antidepressant mianserin and its interaction with various opioid receptor subtypes was evaluated. Mice were tested with a hotplate analgesia meter. Mianserin elicited an antinociceptive effect in a dose-dependent manner following doses from 1-25 mg/kg. As the mianserin dose increased beyond 30 mg/kg, latencies returned to baseline, yielding a biphasic effect. This effect of mianserin was antagonized by naloxone (P<0.005), implying a possible opioid mechanism of action involved in the mianserin induced antinociceptive effect. When administered with various opioid antagonists, the sensitivity of mianserin to selective opioid antagonists was found significant for mu and kappa1 opioid receptor subtypes (P<0.005), but not for delta-receptor. At the next stage mianserin was administered together with various agonists of opioid receptors. When administered together with opiates, mianserin significantly potentiates analgesia at the mu, kappa1 and kappa3 opioid receptor subtype (P<0.005) and to a lesser extent, at the delta opioid receptors. These results suggest a potential use of mianserin in the management of some pain syndromes. However, further research is needed in order to establish both the exact clinical indications and the effective doses of mianserin when prescribed for pain.
Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties.[Pubmed:23301527]
J Med Chem. 2013 Feb 14;56(3):1211-27.
The isoxazol-3-one tautomer of the bicyclic isoxazole, 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol (THAZ), has previously been shown to be a weak GABA(A) and glycine receptor antagonist. In the present study, the potential in this scaffold has been explored through the synthesis and pharmacological characterization of a series of N- and O-substituted THAZ analogues. The analogues N-Bn-THAZ (3d) and O-Bn-THAZ (4d) were found to be potent agonists of the human 5-HT(2A) and 5-HT(2C) receptors. Judging from an elaborate pharmacological profiling at numerous other CNS targets, the 3d analogue appears to be selective for the two receptors. Administration of 3d substantially improved the cognitive performance of mice in a place recognition Y-maze model, an effect fully reversible by coadministration of the selective 5-HT(2C) antagonist SB242084. In conclusion, as novel bioavailable cognitive enhancers that most likely mediate their effects through 5-HT(2A) and/or 5-HT(2C) receptors, the isoxazoles 3d and 4d constitute interesting leads for further medicinal chemistry development.
International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin).[Pubmed:7938165]
Pharmacol Rev. 1994 Jun;46(2):157-203.
It is evident that in the last decade or so, a vast amount of new information has become available concerning the various 5-HT receptor types and their characteristics. This derives from two main research approaches, operational pharmacology, using selective ligands (both agonists and antagonists), and, more recently, molecular biology. Although the scientific community continues to deliberate about the hierarchy of criteria for neurotransmitter receptor characterisation, there seems good agreement between the two approaches regarding 5-HT receptor classification. In addition, the information regarding transduction mechanisms and second messengers is also entirely consistent. Thus, on the basis of these essential criteria for receptor characterisation and classification, there are at least three main groups or classes of 5-HT receptor: 5-HT1, 5-HT2, and 5-HT3. Each group is not only operationally but also structurally distinct, with each receptor group having its own distinct transducing system. The more recently identified 5-HT4 receptor almost undoubtedly represents a fourth 5-HT receptor class on the basis of operational and transductional data, but this will only be definitively shown when the cDNA for the receptor has been cloned and the amino acid sequence of the protein is known. Although those 5-HT receptors that have been fully characterised and classified to date (and, hence, named with confidence) would seem to mediate the majority of the actions of 5-HT throughout the mammalian body, not all receptors for 5-HT are fully encompassed within our scheme of classification. These apparent anomalies must be recognised and need further study. They may or may not represent new groups of 5-HT receptor or subtypes of already known groups of 5-HT receptor. Even though the cDNAs for the 5-ht1E, 5-ht1F, 5-ht5, 5-ht6, and 5-ht7 receptors have been cloned and their amino acid sequence defined, more data are necessary concerning their operational and transductional characteristics before one can be confident of the suitability of their appellations. Therefore, it is important to rationalise in concert all of the available data from studies involving both operational approaches of the classical pharmacological type and those from molecular and cellular biology.(ABSTRACT TRUNCATED AT 400 WORDS)


