PF-03716556Acid pump antagonist,potent and selective CAS# 928774-43-0 |
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- Istaroxime
Catalog No.:BCC1660
CAS No.:203737-93-3
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 928774-43-0 | SDF | Download SDF |
| PubChem ID | 25134521 | Appearance | Powder |
| Formula | C22H26N4O3 | M.Wt | 394.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 23 mg/mL (58.31 mM; Need ultrasonic and warming) | ||
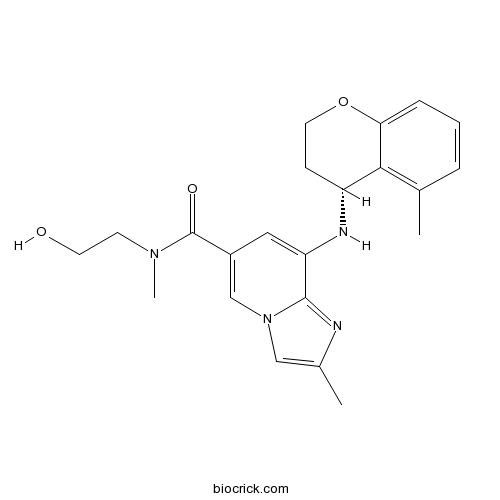
| Chemical Name | N-(2-hydroxyethyl)-N,2-dimethyl-8-[[(4R)-5-methyl-3,4-dihydro-2H-chromen-4-yl]amino]imidazo[1,2-a]pyridine-6-carboxamide | ||
| SMILES | CC1=C2C(CCOC2=CC=C1)NC3=CC(=CN4C3=NC(=C4)C)C(=O)N(C)CCO | ||
| Standard InChIKey | YBHKBMJREUZHOV-QGZVFWFLSA-N | ||
| Standard InChI | InChI=1S/C22H26N4O3/c1-14-5-4-6-19-20(14)17(7-10-29-19)24-18-11-16(22(28)25(3)8-9-27)13-26-12-15(2)23-21(18)26/h4-6,11-13,17,24,27H,7-10H2,1-3H3/t17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | H+,K+-ATPase inhibitor (pIC50 = 6 in human recombinant ion-leaky assays); more potent in acidic conditions. Highly selective for H+,K+-ATPase in vitro; displays no activity at Na+,K+-ATPase. Also displays selectivity for H+,K+-ATPase over a range of 50 receptors and ion channels (IC50 > 10 μM). Inhibits gastric acid secretion in rat and dog models. |

PF-03716556 Dilution Calculator

PF-03716556 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.535 mL | 12.6749 mL | 25.3498 mL | 50.6997 mL | 63.3746 mL |
| 5 mM | 0.507 mL | 2.535 mL | 5.07 mL | 10.1399 mL | 12.6749 mL |
| 10 mM | 0.2535 mL | 1.2675 mL | 2.535 mL | 5.07 mL | 6.3375 mL |
| 50 mM | 0.0507 mL | 0.2535 mL | 0.507 mL | 1.014 mL | 1.2675 mL |
| 100 mM | 0.0253 mL | 0.1267 mL | 0.2535 mL | 0.507 mL | 0.6337 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: In porcine ion-tight membrane vesicles, PF-03716556 inhibited H+,K+-ATPase activity in a concentration-dependent manner, with a pIC50 value of 7.095 ± 0.077 at pH 7.4.
The gastric H+,K+-ATPase, which is responsible for gastric acid secretion, is a P2-type ATPase located in the apical membrane of parietal cells. Inhibition of the H+,K+-ATPase is currently the most effective way to control gastric acid secretion and remains an attractive target for the medical treatment of acidrelated diseases. PF-03716556 is a novel, potent, and selective acid pump antagonist for the treatment of gastroesophageal reflux disease.
In vitro: PF-03716556 demonstrated 3-fold greater inhibitory activity than revaprazan, the only acid pump antagonist that has been available on the market, in ion-tight assay. Kinetics experiments revealed that PF-03716556 has a competitive and reversible mode of action [1].
In vivo: PF-03716556 did not display any species differences, exhibiting highly selective profile including the canine kidney H+,K+-ATPase. In addition, more rapid onset of action than omeprazole and 3-fold greater potency than revaprazan were observed in Ghosh-Schild rats and Heidenhain pouch dogs [2].
Clinical trials: Currenlty no clinical data are available.
Reference:
[1] Mori H, Tonai-Kachi H, Ochi Y, Taniguchi Y, Ohshiro H, Takahashi N, Aihara T, Hirao A, Kato T, Sakakibara M, Kurebayashi Y. N-(2-hydroxyethyl)-N,2-dimethyl-8-{[(4R)-5-methyl-3,4- dihydro-2H-chromen-4-yl]amino}imidazo[1,2-a]pyridine-6-carboxamide (PF-03716556), a novel, potent, and selective acid pump antagonist for the treatment of gastroesophageal reflux disease. J Pharmacol Exp Ther. 2009;328(2):671-9.
- IRAK inhibitor 2
Catalog No.:BCC1655
CAS No.:928333-30-6
- AS 1892802
Catalog No.:BCC6335
CAS No.:928320-12-1
- MN 64
Catalog No.:BCC6489
CAS No.:92831-11-3
- Boc-D-Asp-OBzl
Catalog No.:BCC3370
CAS No.:92828-64-3
- 3-Chloro-1-(4-octylphenyl)-propanone
Catalog No.:BCN2249
CAS No.:928165-59-7
- Tenacissoside F
Catalog No.:BCN4472
CAS No.:928151-78-4
- Alisol O
Catalog No.:BCN3362
CAS No.:928148-51-0
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- RAF265
Catalog No.:BCC3677
CAS No.:927880-90-8
- DPNI-caged-GABA
Catalog No.:BCC5957
CAS No.:927866-58-8
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
- Glochicoccin D
Catalog No.:BCN4471
CAS No.:927812-23-5
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
- Pracinostat (SB939)
Catalog No.:BCC2152
CAS No.:929016-96-6
- GSK461364
Catalog No.:BCC3788
CAS No.:929095-18-1
- 7-Oxo-ganoderic acid Z
Catalog No.:BCN7973
CAS No.:929248-72-6
- LUF 6283
Catalog No.:BCC6318
CAS No.:92933-48-7
- Fmoc-Tyr-OH
Catalog No.:BCC3562
CAS No.:92954-90-0
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
- Sessilifoline A
Catalog No.:BCN4473
CAS No.:929637-35-4
- Cucumegastigmane I
Catalog No.:BCN4474
CAS No.:929881-46-9
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
- 3,4-Dimethoxybenzyl Alcohol
Catalog No.:BCN2721
CAS No.:93-03-8
N-(2-hydroxyethyl)-N,2-dimethyl-8-{[(4R)-5-methyl-3,4-dihydro-2H-chromen-4-yl]ami no}imidazo[1,2-a]pyridine-6-carboxamide (PF-03716556), a novel, potent, and selective acid pump antagonist for the treatment of gastroesophageal reflux disease.[Pubmed:18981288]
J Pharmacol Exp Ther. 2009 Feb;328(2):671-9.
Inhibition of H(+),K(+)-ATPase is accepted as the most effective way of controlling gastric acid secretion. However, current acid suppressant therapy for gastroesophageal reflux disease, using histamine H(2) receptor antagonists and proton pump inhibitors, does not fully meet the needs of all patients because of their mechanism of action. This study sought to characterize the in vitro and in vivo pharmacology of a novel acid pump antagonist, N-(2-Hydroxyethyl)-N,2-dimethyl-8-{[(4R)-5-methyl-3,4-dihydro-2H-chromen-4-yl]ami no}imidazo[1,2-a]pyridine-6-carboxamide (PF-03716556), and to compare it with other acid suppressants. Porcine, canine, and human recombinant gastric H(+),K(+)-ATPase activities were measured by ion-leaky and ion-tight assay. The affinities for a range of receptors, ion channels, and enzymes were determined to analyze selectivity profile. Acid secretion in Ghosh-Schild rats and Heidenhain pouch dogs were measured by titrating perfusate and gastric juice samples. PF-03716556 demonstrated 3-fold greater inhibitory activity than 5,6-dimethyl-2-(4-fluorophenylamino)-4-(1-methyl-1,2,3,4-tetrahydroisoquinoline-2 -yl)pyrimidine (revaprazan), the only acid pump antagonist that has been available on the market, in ion-tight assay. The compound did not display any species differences, exhibiting highly selective profile including the canine kidney Na(+),K(+)-ATPase. Kinetics experiments revealed that PF-03716556 has a competitive and reversible mode of action. More rapid onset of action than 5-methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]-sulfinyl}-benzimidazole (omeprazole) and 3-fold greater potency than revaprazan were observed in Ghosh-Schild rats and Heidenhain pouch dogs. PF-03716556, a novel acid pump antagonist, could improve upon or even replace current pharmacological treatment for gastroesophageal reflux disease.


