AllopurinolCAS# 315-30-0 |
- MK-4305
Catalog No.:BCC1760
CAS No.:1030377-33-3
- SB-408124 Hydrochloride
Catalog No.:BCC1929
CAS No.:1431697-90-3
- SB-674042
Catalog No.:BCC1931
CAS No.:483313-22-0
- TCS 1102
Catalog No.:BCC4063
CAS No.:916141-36-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 315-30-0 | SDF | Download SDF |
| PubChem ID | 2094 | Appearance | Powder |
| Formula | C5H4N4O | M.Wt | 136.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 14 mg/mL (102.86 mM; Need ultrasonic and warming) H2O : 1 mg/mL (7.35 mM; ultrasonic and adjust pH to 11 with NaOH) | ||
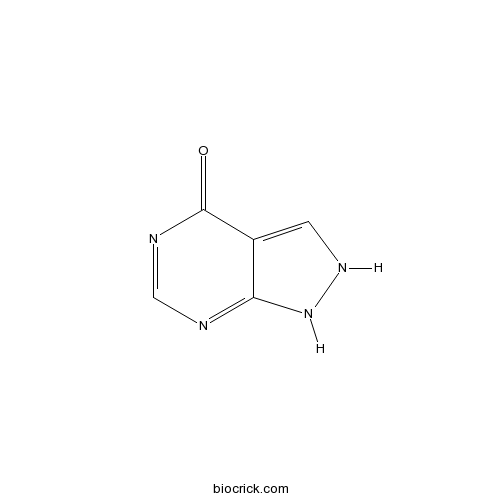
| Chemical Name | 1,2-dihydropyrazolo[3,4-d]pyrimidin-4-one | ||
| SMILES | C1=C2C(=NC=NC2=O)NN1 | ||
| Standard InChIKey | OFCNXPDARWKPPY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Allopurinol (Zyloprim) is a xanthine oxidase inhibitor with an IC50 of 7.82±0.12 μM.
Target: XAO
Allopurinol (Zyloprim, and generics) is a drug used primarily to treat hyperuricemia (excess uric acid in blood plasma) and its complications, including chronic gout. It is a xanthine oxidase inhibitor which is administered orally. A common misconception is that allopurinol is metabolized by its target, xanthine oxidase, but this action is principally carried out by Aldehyde oxidase. The active metabolite of allopurinol is oxypurinol, which is also an inhibitor of xanthine oxidase. Allopurinol is almost completely metabolized to oxypurinol within two hours of oral administration, whereas oxypurinol is slowly excreted by the kidneys over 18–30 hours. For this reason, oxypurinol is believed responsible for the majority of allopurinol's effect.
Allopurinol is a purine analog; it is a structural isomer of hypoxanthine (a naturally occurring purine in the body) and is an inhibitor of the enzyme xanthine oxidase. In addition to blocking uric acid production, inhibition of xanthine oxidase causes an increase in hypoxanthine and xanthine. While xanthine cannot be converted to purine ribotides, hypoxanthine can be salvaged to the purine ribotides adenosine and guanosine monophosphates. Increased levels of these ribotides may cause feedback inhibition of amidophosphoribosyl transferase, the first and rate-limiting enzyme of purine biosynthesis. Allopurinol, therefore, decreases uric acid formation and may also inhibit purine synthesis. References: | |||||

Allopurinol Dilution Calculator

Allopurinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.347 mL | 36.735 mL | 73.47 mL | 146.94 mL | 183.675 mL |
| 5 mM | 1.4694 mL | 7.347 mL | 14.694 mL | 29.388 mL | 36.735 mL |
| 10 mM | 0.7347 mL | 3.6735 mL | 7.347 mL | 14.694 mL | 18.3675 mL |
| 50 mM | 0.1469 mL | 0.7347 mL | 1.4694 mL | 2.9388 mL | 3.6735 mL |
| 100 mM | 0.0735 mL | 0.3673 mL | 0.7347 mL | 1.4694 mL | 1.8367 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
An anti-urolithic Xanthine oxidase inhibitor that decreases uric acid production and is used in treatment of hyperuricemia and chronic gout.
- Crotaline
Catalog No.:BCN4983
CAS No.:315-22-0
- Sunifiram
Catalog No.:BCC4167
CAS No.:314728-85-3
- 6-Methoxysalicylic Acid
Catalog No.:BCC8288
CAS No.:3147-64-6
- Mebendazole
Catalog No.:BCC9016
CAS No.:31431-39-7
- 4-Amino-3-nitrobenzophenone
Catalog No.:BCC8682
CAS No.:31431-19-3
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- Isotachioside
Catalog No.:BCN5230
CAS No.:31427-08-4
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
- BPTES
Catalog No.:BCC6506
CAS No.:314045-39-1
- (R)-(-)-Apomorphine hydrochloride
Catalog No.:BCC7250
CAS No.:314-19-2
- Evans Blue tetrasodium salt
Catalog No.:BCC6815
CAS No.:314-13-6
- VDM 11
Catalog No.:BCC7044
CAS No.:313998-81-1
- Testosterone enanthate
Catalog No.:BCC9169
CAS No.:315-37-7
- Acetylheliosupine
Catalog No.:BCN1981
CAS No.:31514-30-4
- PAC-1
Catalog No.:BCC3600
CAS No.:315183-21-2
- Ifflaiamine
Catalog No.:BCN7061
CAS No.:31520-95-3
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
- Isobavachin
Catalog No.:BCN5232
CAS No.:31524-62-6
- 5-Hydroxyseselin
Catalog No.:BCN3428
CAS No.:31525-75-4
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
- Matsukaze-lactone
Catalog No.:BCN7580
CAS No.:3153-73-9
- 1,18-Octadecanediol
Catalog No.:BCN5233
CAS No.:3155-43-9
- Kavain
Catalog No.:BCN8295
CAS No.:3155-48-4
- TC-DAPK 6
Catalog No.:BCC1989
CAS No.:315694-89-4
Cost-effectiveness Analysis for Genotyping before Allopurinol Treatment to Prevent Severe Cutaneous Adverse Drug Reactions.[Pubmed:28365572]
J Rheumatol. 2017 Jun;44(6):835-843.
OBJECTIVE: Patients with an HLA-B*58:01 allele have an increased risk of developing severe cutaneous adverse drug reactions (SCAR) when treated with Allopurinol. Although one-off pharmacogenetic testing may prevent life-threatening adverse drug reactions, testing prior to Allopurinol initiation incurs additional costs. The study objective was to evaluate the cost-effectiveness of HLA-B*58:01 screening compared with using other available urate-lowering agents (ULA). METHODS: A decision-analytical model was used to compare direct medical costs and effectiveness [including lifetime saved, quality-adjusted life-yrs (QALY) gained] in treating new patients with the following options: (1) genetic screening followed by Allopurinol prescribing for noncarriers of HLA-B*58:01, (2) prescribing benzbromarone without screening, (3) prescribing febuxostat without screening, and (4) prescribing Allopurinol without screening. A 1-year time frame and third-party payer perspective were modeled for both the entire cohort (base-case) and for the subgroup of patients with chronic kidney disease (CKD). RESULTS: The incremental cost-effectiveness ratio of genetic screening prior to ULA therapy was estimated as New Taiwan (NT) $234,610 (US$7508) per QALY gained in the base-case cohort. For patients with CKD, it was estimated as NT$230,925 (US$7390) per QALY. The study results were sensitive to the probability of benzbromarone/febuxostat-related hypersensitivity, and a negative predicted value of genotyping. CONCLUSION: HLA-B*58:01 screening gave good value for money in preventing Allopurinol-induced SCAR in patients indicated for ULA therapy. In addition to the costs of genotyping, it is important to monitor ULA safety closely in adopting HLA-B*58:01 screening in practice.
Role of Allopurinol in Optimizing Thiopurine Therapy in Patients with Autoimmune Hepatitis: A Review.[Pubmed:28348471]
J Clin Exp Hepatol. 2017 Mar;7(1):55-62.
Autoimmune hepatitis (AIH) is a chronic immune mediated liver disease characterized by elevated transaminases, hyper gammaglobulinemia, presence of autoantibodies and interface hepatitis in the absence of a known etiology of liver disease. Thiopurines (azathioprine [AZA]/6-mercaptopurine [6MP]) and steroids remain the first line of treatment of AIH in both children and adults. However, a small proportion of AIH patients are either non-responders or develop side effects with AZA. The metabolism of AZA is complex and mediated by multiple enzymes. After absorption and getting converted to 6MP, it is converted to 6-thiouric acid, 6-methyl mercaptopurine (6MMP) and 6-thioguanine (6TG) by different enzymes. Elevated 6MMP levels are associated with hepatotoxicity and also poor efficacy due to simultaneous lower levels of 6TG, which is the active drug metabolite related to both efficacy and myelosuppression. Allopurinol, a xanthine oxidase inhibitor shifts the metabolism of AZA away from 6MMP toward 6TG. This combination of Allopurinol with reduced dose of AZA is an alternative to more expensive and toxic second line therapy to induce remission in patients with AIH. This article discusses the mechanism of action of Allopurinol in inducing response to AZA, reviews the published literature on this combination therapy and gives guidelines on the use of Allopurinol in patients with AIH.
N-acetyl cysteine versus allopurinol in the prevention of contrast nephropathy in patients with chronic kidney disease: A randomized controlled trial.[Pubmed:28356658]
Indian J Nephrol. 2017 Mar-Apr;27(2):93-98.
Contrast media administration can lead to acute deterioration in renal function particularly in patients with pre-existing chronic kidney disease. This prospective, randomized controlled open-label parallel group study was undertaken at Nizam's Institute of Medical Sciences, Hyderabad, from June to December 2015. A total of 95 patients were included, of which 35 received n-acetylcysteine (NAC) + normal saline (NS), 30 patients received Allopurinol (ALL) + NS, and 30 patients received placebo. In our study, the overall incidence of CIN was 24%. Incidence of CIN in NAC + NS, ALL + NS, and placebo group were 20%, 16%, and 36%, respectively. The major finding of this study was there was no significant difference between NAC and Allopurinol in the prevention of contrast nephropathy. However, only Allopurinol was superior to placebo. In our study, hyperuricemia and baseline serum creatinine were the only risk factors associated with CIN.
Allopurinol and the risk of ventricular arrhythmias in the elderly: a study using US Medicare data.[Pubmed:28327188]
BMC Med. 2017 Mar 22;15(1):59.
BACKGROUND: There are no published human studies investigating whether the use of Allopurinol, the most commonly used medication for the treatment of hyperuricemia in gout, the most common type of inflammatory arthritis in adults, has any beneficial effects on ventricular electrophysiology. The objective of our study was to assess whether Allopurinol use is associated with a reduction in the risk of ventricular arrhythmias (VA). METHODS: We used the 5% random sample of Medicare beneficiaries from 2006-2012 to examine new Allopurinol use and the risk of incident VA. Multivariable Cox regression analyses were adjusted for demographics (age, race, sex), comorbidity, cardiac medications, and conditions associated with VA. We calculated hazard ratios (HR) and 95% confidence intervals (CI). RESULTS: Of the 28,755 episodes of new Allopurinol use, 2538 were associated with incident VA (8.8%). Among patients with incident VA, 54% were male, 78% were White, 75% had gout as the underlying diagnosis, and the mean Charlson-Romano comorbidity score was 4.8. The crude incidence of VA per 1,000,000 person-days declined as the duration of Allopurinol use increased: 1-180 days, 151; 181 days to 2 years, 105; and > 2 years, 85. In multivariable-adjusted analyses, compared to non-use, Allopurinol use was associated with lower HR of VA of 0.82 (95% CI, 0.76-0.90). Compared to Allopurinol non-use, longer Allopurinol use durations were significantly associated with lower multivariable-adjusted HR for VA: 1-180 days, 0.96 (95% CI, 0.85-1.08); 181 days to 2 years, 0.76 (95% CI, 0.68-0.85); and > 2 years, 0.72 (95% CI, 0.60-0.87). Multiple sensitivity analyses adjusting for cardiac conditions, anti-arrhythmic drugs and alternate definitions confirmed our findings with minimal/no attenuation of estimates. CONCLUSION: Allopurinol use and use duration of more than 6 months were independently associated with a lower risk of VA. Future studies need to assess the pathophysiology of this potential benefit.


