PRIMA-1BAX inhibitor CAS# 5608-24-2 |
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- Bax inhibitor peptide V5
Catalog No.:BCC2394
CAS No.:579492-81-2
Quality Control & MSDS
Number of papers citing our products

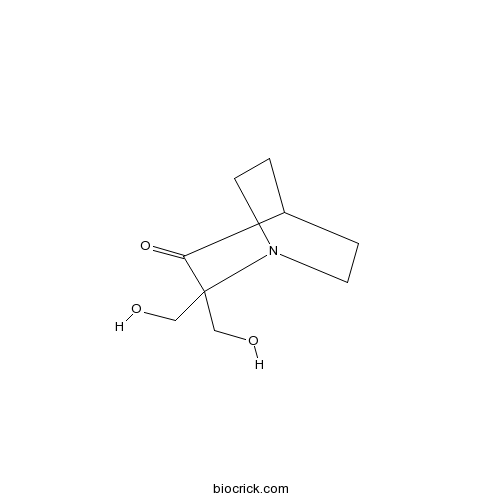
Chemical structure
3D structure
| Cas No. | 5608-24-2 | SDF | Download SDF |
| PubChem ID | 322968 | Appearance | Powder |
| Formula | C9H15NO3 | M.Wt | 185.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (269.95 mM; Need ultrasonic) | ||
| Chemical Name | 2,2-bis(hydroxymethyl)-1-azabicyclo[2.2.2]octan-3-one | ||
| SMILES | C1CN2CCC1C(=O)C2(CO)CO | ||
| Standard InChIKey | RFBVBRVVOPAAFS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H15NO3/c11-5-9(6-12)8(13)7-1-3-10(9)4-2-7/h7,11-12H,1-6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selectively restores mutant p53 activity in tumor cells via activation of Bax and PUMA. Induces apoptosis and inhibits growth of tumors with mutant p53. |

PRIMA-1 Dilution Calculator

PRIMA-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.399 mL | 26.9949 mL | 53.9898 mL | 107.9797 mL | 134.9746 mL |
| 5 mM | 1.0798 mL | 5.399 mL | 10.798 mL | 21.5959 mL | 26.9949 mL |
| 10 mM | 0.5399 mL | 2.6995 mL | 5.399 mL | 10.798 mL | 13.4975 mL |
| 50 mM | 0.108 mL | 0.5399 mL | 1.0798 mL | 2.1596 mL | 2.6995 mL |
| 100 mM | 0.054 mL | 0.2699 mL | 0.5399 mL | 1.0798 mL | 1.3497 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: The value varied among different tumor type. In Saos-2-His-273 cells, PRIMA-1 induced cell death with an IC50 of over 15 M.
PRIMA-1, a novel low molecular weight compound, rescues the tumor-suppressing function of mutant p53 proteins and shows anti-tumor activity in vivo. P53 severs as a crucial tumor suppressor and mutant p53 is expressed at high levels in many tumors. PRIMA-1 is considered as a lead compound for the development of anticancer drugs targeting mutant p53. [1]
In vitro: The substantial increase in Saos-2-His-273-cells death could be noticed after being treated with 125 μM PRIMA-1 for 48 hours. TUNEL staining revealed that such tumor-cell death was primarily triggered by apoptosis. PRIMA-1 could also restore the transcriptional transactivation function to mutant p53 in vitro. [2]
In vivo: To assess the effect of PRIMA-1 on human tumor xenografts, mice were inoculated with Saos-2-His-273 cells expressing mutant p53. The mice then received PRIMA-1 treatment at intra-tumor does of 20 mg/kg or i.v. doses of 20 and 100 mg/kg twice a day for three days. Compared with the control group, the average tumor volume decreased from 555.7 mm3 to 11.7 (100 mg/kg) and 53 (20 mg/kg) mm3 after i.v. injections of PRIMA-1. Intra-tumor injections of PRIMA-1 also decreased the average tumor volume to 5.3 mm3. [2]
Clinical trial: The methylated form of PRIMA-1, PRIMA-1MET was tested on 22 patients with hematologic malignancies and prostate cancer. Based on the clinical data, PRIMA-1MET was safe at predicted therapeutic dose, had a favorable pharmacokinetic profile and could lead to apoptosis of tumor cells in the p53–dependent manner. [3]
References:
[1] Bykov V, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J, Selivanova G, Wiman KG. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem. 2005 Aug; 280(34): 30384–91.
[2] Bykov V, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002 Mar; 8(3):282-8.
[3] Lehmann S, Bykov V, Ali D, Andren O, Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A, Paul C, Wiman KG, Andersson PO. Targeting p53 in vivo: A first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012. DOI: 10.1200/JCO.2011.40.7783.
- Hispidin
Catalog No.:BCN3567
CAS No.:56070-89-4
- Sucralose
Catalog No.:BCC4725
CAS No.:56038-13-2
- Eburicoic acid
Catalog No.:BCN2556
CAS No.:560-66-7
- 9-Hydroxy-4-androstene-3,17-dione
Catalog No.:BCC8802
CAS No.:560-62-3
- Chlorhexidine acetate
Catalog No.:BCC8912
CAS No.:56-95-1
- Histamine 2HCl
Catalog No.:BCC4530
CAS No.:56-92-8
- (H-Cys-OH)2
Catalog No.:BCC2915
CAS No.:56-89-3
- L-lysine
Catalog No.:BCN7157
CAS No.:56-87-1
- L-Glutamic acid
Catalog No.:BCN3809
CAS No.:56-86-0
- L-Glutamine
Catalog No.:BCC3803
CAS No.:56-85-9
- H-Asp-OH
Catalog No.:BCC2881
CAS No.:56-84-8
- Glycerol
Catalog No.:BCC8990
CAS No.:56-81-5
- 3-Methoxyshancigusin I
Catalog No.:BCC9002
CAS No.:
- Ionomycin free acid
Catalog No.:BCC7261
CAS No.:56092-81-0
- Ionomycin calcium salt
Catalog No.:BCC5805
CAS No.:56092-82-1
- Securinine
Catalog No.:BCN6988
CAS No.:5610-40-2
- Parisaponin I
Catalog No.:BCN2835
CAS No.:561007-63-4
- Asperglaucide
Catalog No.:BCN5748
CAS No.:56121-42-7
- 4-O-Methylhelichrysetin
Catalog No.:BCN3986
CAS No.:56121-44-9
- Valrubicin
Catalog No.:BCC5219
CAS No.:56124-62-0
- H-Sar-OtBu.HCl
Catalog No.:BCC3336
CAS No.:5616-81-9
- Acarbose
Catalog No.:BCC1190
CAS No.:56180-94-0
- CGP 13501
Catalog No.:BCC7097
CAS No.:56189-68-5
- Terpinine-4-ol
Catalog No.:BCN8250
CAS No.:562-74-3
Hypoxic resistance of KRAS mutant tumor cells to 3-Bromopyruvate is counteracted by Prima-1 and reversed by N-acetylcysteine.[Pubmed:27863474]
BMC Cancer. 2016 Nov 18;16(1):902.
BACKGROUND: The metabolic inhibitor 3-bromopyruvate (3-BrPA) is a promising anti-cancer alkylating agent, shown to inhibit growth of some colorectal carcinoma with KRAS mutation. Recently, we demonstrated increased resistance to 3-BrPA in wt p53 tumor cells compared to those with p53 silencing or mutation. Since hypoxic microenvironments select for tumor cells with diminished therapeutic response, we investigated whether hypoxia unequally increases resistance to 3-BrPA in wt p53 MelJuso melanoma harbouring (Q61L)-mutant NRAS and wt BRAF, C8161 melanoma with (G12D)-mutant KRAS (G464E)-mutant BRAF, and A549 lung carcinoma with a KRAS (G12S)-mutation. Since hypoxia increases the toxicity of the p53 activator, PRIMA-1 against breast cancer cells irrespective of their p53 status, we also investigated whether PRIMA-1 reversed hypoxic resistance to 3-BrPA. RESULTS: In contrast to the high susceptibility of hypoxic mutant NRAS MelJuso cells to 3-BrPA or PRIMA-1, KRAS mutant C8161 and A549 cells revealed hypoxic resistance to 3-BrPA counteracted by PRIMA-1. In A549 cells, PRIMA-1 increased p21CDKN1mRNA, and reciprocally inhibited mRNA expression of the SLC2A1-GLUT1 glucose transporter-1 and ALDH1A1, gene linked to detoxification and stem cell properties. 3-BrPA lowered CAIX and VEGF mRNA expression. Death from joint PRIMA-1 and 3-BrPA treatment in KRAS mutant A549 and C8161 cells seemed mediated by potentiating oxidative stress, since it was antagonized by the anti-oxidant and glutathione precursor N-acetylcysteine. CONCLUSIONS: This report is the first to show that PRIMA-1 kills hypoxic wt p53 KRAS-mutant cells resistant to 3-BrPA, partly by decreasing GLUT-1 expression and exacerbating pro-oxidant stress.
PRIMA-1 targets the vulnerability of multiple myeloma of deregulated protein homeostasis through the perturbation of ER stress via p73 demethylation.[Pubmed:27533450]
Oncotarget. 2016 Sep 20;7(38):61806-61819.
Despite therapeutic advancement, multiple myeloma (MM) remains incurable with drug resistance being one of the main challenges in the clinic. Myeloma cells possess high protein secretory load, leading to increased intracellular endoplasmic reticulum (ER) stress. Hence, they are vulnerable to further perturbation to its protein homeostasis. In studying the therapeutic mechanism of PRIMA-1 (mutant-p53-reactivating-agent), we uncovered its novel p53-independent-mechanism that can be exploited for myeloma. Despite its inability in restoring the wild type-p53 protein conformation and transcriptional function in the mutant-p53-human-myeloma-cells, PRIMA-1 was efficacious against myeloma cells with differential p53 genotypes. Strikingly, cells without p53 expression demonstrated highest drug sensitivity. Genome-wide gene-expression analysis revealed the involvement of ER stress/UPR-pathway in inducing PRIMA-1-toxicity. UPR markers, HSP70, CHOP and GADD34, were significantly up-regulated, concomitantly with the induction of apoptosis. Furthermore, there was a global attenuation of protein synthesis, correlated with phospho-eIF2a up-regulation. Mechanistically, we identified that PRIMA-1 could cause the demethylation of TP73, through DNMT1 depletion, to subsequently enhance UPR. Of clinical significance, we observed that PRIMA-1 had additive therapeutic effects with another UPR-inducing-agent, bortezomib. Importantly, it can partially re-sensitize bortezomib-resistant cells to bortezomib. Given that MM is already stressed at the baseline in the ER, our results implicated that PRIMA-1 is a potential therapeutic option in MM by targeting its Achilles heel.
PRIMA-1 induces caspase-mediated apoptosis in acute promyelocytic leukemia NB4 cells by inhibition of nuclear factor-kappaB and downregulation of Bcl-2, XIAP, and c-Myc.[Pubmed:27548348]
Anticancer Drugs. 2017 Jan;28(1):51-58.
Restoration of p53 function triggers cell death and eliminates tumors in vivo. Identification of p53-reactivating small molecules such as PRIMA-1 holds promise for effective new anticancer therapies. Here, we investigated the effects of small molecule PRIMA-1 on cell viability and expression of p53-regulated genes and proteins in the acute promyelocytic leukemia-derived NB4 cell line. Our results showed that PRIMA-1 had antileukemic properties in acute promyelocytic leukemia-derived NB4 cells. PRIMA-1-triggered apoptosis in a dose-dependent and time-dependent manner as indicated by the MTT assay and annexin-V staining. Apoptosis induction by PRIMA-1 was associated with caspase-9, caspase-7 activation and PARP cleavage. p21 protein expression was increased after PRIMA-1 treatment and real-time PCR analysis of proapoptotic p53 target genes indicated upregulation of Bax and Noxa. Western blot analysis showed that IkappaBalpha phosphorylation and its degradation were inhibited by PRIMA-1. Moreover, protein expression of nuclear factor-kappaB-regulated antiapoptotic (Bcl-2 and XIAP) and proliferative (c-Myc) gene products was decreased. Importantly, PRIMA-1 did not show any significant apoptotic effect in normal human peripheral blood mononuclear cells. These in-vitro studies imply that p53 reactivation by small compounds may become a novel anticancer therapy in acute promyelocytic leukemia.
APR-246 (PRIMA-1(MET)) strongly synergizes with AZD2281 (olaparib) induced PARP inhibition to induce apoptosis in non-small cell lung cancer cell lines.[Pubmed:26975633]
Cancer Lett. 2016 Jun 1;375(2):313-322.
APR-246 (PRIMA-1(Met)) is able to bind mutant p53 and restore its normal conformation and function. The compound has also been shown to increase intracellular ROS levels. Importantly, the poly-[ADP-ribose] polymerase-1 (PARP-1) enzyme plays an important role in the repair of ROS-induced DNA damage. We hypothesize that by blocking this repair with the PARP-inhibitor AZD2281 (olaparib), DNA damage would accumulate in the cell leading to massive apoptosis. We observed that APR-246 synergistically enhanced the cytotoxic response of olaparib in TP53 mutant non-small cell lung cancer cell lines, resulting in a strong apoptotic response. In the presence of wild type p53 a G2/M cell cycle block was predominantly observed. NOXA expression levels were significantly increased in a TP53 mutant background, and remained unchanged in the wild type cell line. The combined treatment of APR-246 and olaparib induced cell death that was associated with increased ROS production, accumulation of DNA damage and translocation of p53 to the mitochondria. Out data suggest a promising targeted combination strategy in which the response to olaparib is synergistically enhanced by the addition of APR-246, especially in a TP53 mutant background.
PRIMA-1 induces apoptosis by inhibiting JNK signaling but promoting the activation of Bax.[Pubmed:17113036]
Biochem Biophys Res Commun. 2007 Jan 5;352(1):203-12.
The p53 protein plays a major role in the maintenance of genome stability in mammalian cells. Mutations of p53 occur in over 40% of breast cancers and are indicative of tumor resistance to chemotherapeutic agents. Recently, there has been a high degree of interest in pharmacological approaches for restoring the normal function to mutant p53. The low molecular weight compound p53 reactivation and induction of massive apoptosis (PRIMA-1) was shown to induce cytotoxic effects and apoptosis in human tumor cells with mutant p53. Here, we studied the molecular mechanisms of PRIMA-1-induced apoptosis in human breast cancer cells with p53 mutations such as MDA-231 and GI-101A as compared to MCF-7 cells. We show that PRIMA-1 selectively induces apoptosis in human breast cancer cells MDA-231 and GI-101A compared to the MCF-7. This effect was paralleled by an increase in total p53 level in the nucleus and the induction of its phosphorylation at Ser-15 site. Using the chromatin immunoprecipitation (ChIP) assays, we show that PRIMA-1 restored p53 DNA binding activity to the promoters of the proapoptotic genes such as Bax and PUMA, but inhibited the binding activity to the promoters of the MAP4K4 gene. Knockdown of p53 protein in breast cancer cells using siRNA followed by PRIMA-1 treatment resulted in decline of Bax and PUMA proteins expression. Cell incubation with either PRIMA-1 or SP600125 (c-Jun NH2-terminal kinase inhibitor) resulted in the abrogation of adriamycin-induced c-Jun NH2-terminal kinase (JNK) activation, whereas Bax activation was not inhibited. We conclude that both Bax and PUMA but not JNK signaling are involved in PRIMA-1-induced apoptosis in breast cancer cells with p53 mutation.
Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound.[Pubmed:11875500]
Nat Med. 2002 Mar;8(3):282-8.
The tumor suppressor p53 inhibits tumor growth primarily through its ability to induce apoptosis. Mutations in p53 occur in at least 50% of human tumors. We hypothesized that reactivation of mutant p53 in such tumors should trigger massive apoptosis and eliminate the tumor cells. To test this, we screened a library of low-molecular-weight compounds in order to identify compounds that can restore wild-type function to mutant p53. We found one compound capable of inducing apoptosis in human tumor cells through restoration of the transcriptional transactivation function to mutant p53. This molecule, named PRIMA-1, restored sequence-specific DNA binding and the active conformation to mutant p53 proteins in vitro and in living cells. PRIMA-1 rescued both DNA contact and structural p53 mutants. In vivo studies in mice revealed an antitumor effect with no apparent toxicity. This molecule may serve as a lead compound for the development of anticancer drugs targeting mutant p53.
Mutant p53-dependent growth suppression distinguishes PRIMA-1 from known anticancer drugs: a statistical analysis of information in the National Cancer Institute database.[Pubmed:12507923]
Carcinogenesis. 2002 Dec;23(12):2011-8.
We recently identified PRIMA-1 as a low molecular weight compound that restores tumor suppressor function to mutant p53 proteins and has anti-tumor activity in vivo (1). Here we report the statistical analysis of the effect of PRIMA-1 on a panel of human tumor cell lines using information available in a database at the Developmental Therapeutics Program of the National Cancer Institute (NCI). We extracted growth inhibition profiles for PRIMA-1 and 44 known anticancer agents, p53 status of cell lines, population doubling time, and level of p53 protein expression from the NCI database. The data were analyzed by linear regression, Wilcoxon matched pairs test, and cluster analysis. In a subset of human cell lines derived from colon, ovarian, renal, and non-small cell lung cancer and melanoma, the level of mutant p53 expression correlated with cell population doubling time, r = -0.53, P = 0.018. The GI(50) values for PRIMA-1 correlated with levels of mutant p53, r = -0.75, P = 0.0002. PRIMA-1 showed a statistically significant preference at P = 0.04 for growth inhibition of tumor cell lines expressing mutant p53 as compared with lines expressing wild-type p53. In contrast, none of several known anticancer drugs showed such preference. PRIMA-1 inhibited the growth of cell lines derived from various human tumor types in a mutant p53-dependent manner. This distinguishes PRIMA-1 from known anticancer drugs and supports the idea that PRIMA-1 can serve as a lead for the development of novel therapeutic compounds.


