ALW-II-41-27Eph receptor inhibitor CAS# 1186206-79-0 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1186206-79-0 | SDF | Download SDF |
| PubChem ID | 42628503 | Appearance | Powder |
| Formula | C32H32F3N5O2S | M.Wt | 607.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Eph receptor tyrosine kinase inhibitor | ||
| Solubility | DMSO : ≥ 47 mg/mL (77.34 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
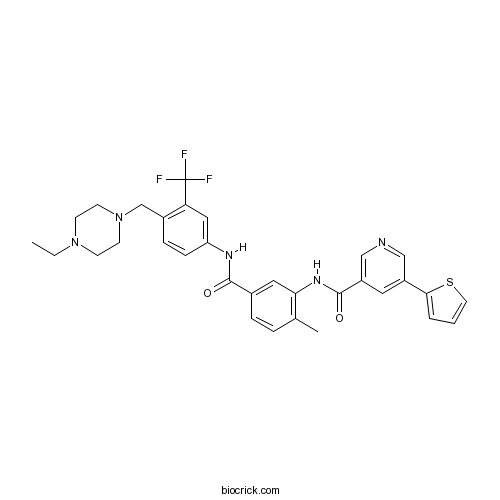
| Chemical Name | N-[5-[[4-[(4-ethylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]carbamoyl]-2-methylphenyl]-5-thiophen-2-ylpyridine-3-carboxamide | ||
| SMILES | CCN1CCN(CC1)CC2=C(C=C(C=C2)NC(=O)C3=CC(=C(C=C3)C)NC(=O)C4=CN=CC(=C4)C5=CC=CS5)C(F)(F)F | ||
| Standard InChIKey | HYWXBDQAYLPMIX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C32H32F3N5O2S/c1-3-39-10-12-40(13-11-39)20-23-8-9-26(17-27(23)32(33,34)35)37-30(41)22-7-6-21(2)28(16-22)38-31(42)25-15-24(18-36-19-25)29-5-4-14-43-29/h4-9,14-19H,3,10-13,20H2,1-2H3,(H,37,41)(H,38,42) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ALW-II-41-27 is a novel Eph receptor tyrosine kinase inhibitor, used for cancer treatment.In Vitro:ALW-II-41-27 inhibits Ba/F3 cells transformed with Tel fusions of EphA3, Kit, Fms, KDR, FLT1, FGR, Src, Lyn, Bmx, and Bcr-Abl with an EC50 below 500 nM. ALW-II-41-27 exhibits cross reactivity with Bcr-Abl. ALW-II-41-27 inhibits b-raf, CSF1R, DDR1, DDR2, EphA2, EphA5, EphA8, EphB1, EphB2, EphB3, Frk, Kit, Lck, p38α, p38β, PDGFRα, PDGFRβ, and Raf1 and many more demonstrating how introduction of the thiophene group can have a tremendous impact on kinase selectivity[1]. References: | |||||

ALW-II-41-27 Dilution Calculator

ALW-II-41-27 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6456 mL | 8.2279 mL | 16.4558 mL | 32.9115 mL | 41.1394 mL |
| 5 mM | 0.3291 mL | 1.6456 mL | 3.2912 mL | 6.5823 mL | 8.2279 mL |
| 10 mM | 0.1646 mL | 0.8228 mL | 1.6456 mL | 3.2912 mL | 4.1139 mL |
| 50 mM | 0.0329 mL | 0.1646 mL | 0.3291 mL | 0.6582 mL | 0.8228 mL |
| 100 mM | 0.0165 mL | 0.0823 mL | 0.1646 mL | 0.3291 mL | 0.4114 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ALW-II-41-27 is a potent inhibitor of EPH family kinases, with an IC50 value of 11 nM to EPHA2 [1] [2].
EPH family proteins are key regulators of both disease and normal development. EPH receptors are involved in many intracellular signaling pathways such as PI3K/AKT/mTOR, RAS/RAF/MAPK, FAK, SRC, ABL, and RHO/RAC/CDC42 [2].
In H358 cells, treatment with ALW-II-41-27 at a concentration of 1 μM within 15 minutes impaired the tyrosine phosphorylation of the EPHA2 receptor and continued to inhibit the tyrosine phosphorylation through 6 hours. ALW-II-41-27 also dose-dependently inhibited the EPHA2 phosphorylation induced by ligand. When the EPHA2 was depleted by RNAi in NSCLC cell lines, cells were much less sensitive to ALW-II-41-27.
It was suggested that EPHA2 plays an oncogenic role according to results in lung cancers. In mice bearing non–small cell lung cancers (NSCLCs), intraperitoneal injection with ALW-II-41-27 at a dose of 15 mg/kg twice daily for 14 days significantly resulted in an inhibition of the growth of H358 tumors. ALW-II-41-27 significantly increased the apoptosis of tumors compared with the vehicle alone or NG-25. This was similar to the effect of the genetic ablation of EPHA2. Compared with treatments with vehicle alone or NG-25, treatment with ALW-II-41-27 did not result in significant differences in the vessel density or proliferation of tumors [2].
References:
[1]. Marialuisa Moccia, Qingsong Liu, Teresa Guida, et al. Identification of Novel Small Molecule Inhibitors of Oncogenic RET Kinase. PLOS ONE, 2015, 10(6):e0128364.
[2]. Katherine R. Amato, Shan Wang, Andrew K. Hastings, et al. Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. Journal of Clinical Investigation, 2014, 124(5):2037-2049.
- NPE-caged-proton
Catalog No.:BCC7698
CAS No.:1186195-63-0
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- Tocrifluor T1117
Catalog No.:BCC7401
CAS No.:1186195-59-4
- BU 226 hydrochloride
Catalog No.:BCC6936
CAS No.:1186195-56-1
- Vitexdoin A
Catalog No.:BCN4089
CAS No.:1186021-77-1
- VD3-D6
Catalog No.:BCC4076
CAS No.:118584-54-6
- NVP-BVU972
Catalog No.:BCC3828
CAS No.:1185763-69-2
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
- Floribundone 1
Catalog No.:BCN4726
CAS No.:118555-84-3
- Phaseoloidin
Catalog No.:BCN8451
CAS No.:118555-82-1
- Boc-Orn(2-Cl-Z)-OH
Catalog No.:BCC3428
CAS No.:118554-00-0
- H-Orn(2-Cl-Z)-OH
Catalog No.:BCC3002
CAS No.:118553-99-4
- 4SC-202
Catalog No.:BCC5359
CAS No.:1186222-89-8
- Epivogeloside
Catalog No.:BCN6060
CAS No.:118627-52-4
- TMN 355
Catalog No.:BCC6121
CAS No.:1186372-20-2
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- 11-Hydroxycodaphniphylline
Catalog No.:BCN6061
CAS No.:1186496-68-3
- H-Cys(Me)-OH
Catalog No.:BCC2908
CAS No.:1187-84-4
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- Cuniloside B
Catalog No.:BCN6062
CAS No.:1187303-40-7
- Trametinib DMSO solvate
Catalog No.:BCC2013
CAS No.:1187431-43-1
- PF 184
Catalog No.:BCC6130
CAS No.:1187460-81-6
- Baricitinib (LY3009104, INCB028050)
Catalog No.:BCC2195
CAS No.:1187594-09-7
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC.[Pubmed:24713656]
J Clin Invest. 2014 May;124(5):2037-49.
Genome-wide analyses determined previously that the receptor tyrosine kinase (RTK) EPHA2 is commonly overexpressed in non-small cell lung cancers (NSCLCs). EPHA2 overexpression is associated with poor clinical outcomes; therefore, EPHA2 may represent a promising therapeutic target for patients with NSCLC. In support of this hypothesis, here we have shown that targeted disruption of EphA2 in a murine model of aggressive Kras-mutant NSCLC impairs tumor growth. Knockdown of EPHA2 in human NSCLC cell lines reduced cell growth and viability, confirming the epithelial cell autonomous requirements for EPHA2 in NSCLCs. Targeting EPHA2 in NSCLCs decreased S6K1-mediated phosphorylation of cell death agonist BAD and induced apoptosis. Induction of EPHA2 knockdown within established NSCLC tumors in a subcutaneous murine model reduced tumor volume and induced tumor cell death. Furthermore, an ATP-competitive EPHA2 RTK inhibitor, ALW-II-41-27, reduced the number of viable NSCLC cells in a time-dependent and dose-dependent manner in vitro and induced tumor regression in human NSCLC xenografts in vivo. Collectively, these data demonstrate a role for EPHA2 in the maintenance and progression of NSCLCs and provide evidence that ALW-II-41-27 effectively inhibits EPHA2-mediated tumor growth in preclinical models of NSCLC.
Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers.[Pubmed:28581527]
Oncogene. 2017 Oct 5;36(40):5620-5630.
Basal-like/triple-negative breast cancers (TNBCs) are among the most aggressive forms of breast cancer, and disproportionally affects young premenopausal women and women of African descent. Patients with TNBC suffer a poor prognosis due in part to a lack of molecularly targeted therapies, which represents a critical barrier for effective treatment. Here, we identify EphA2 receptor tyrosine kinase as a clinically relevant target for TNBC. EphA2 expression is enriched in the basal-like molecular subtype in human breast cancers. Loss of EphA2 function in both human and genetically engineered mouse models of TNBC reduced tumor growth in culture and in vivo. Mechanistically, targeting EphA2 impaired cell cycle progression through S-phase via downregulation of c-Myc and stabilization of the cyclin-dependent kinase inhibitor p27/KIP1. A small molecule kinase inhibitor of EphA2 effectively suppressed tumor cell growth in vivo, including TNBC patient-derived xenografts. Thus, our data identify EphA2 as a novel molecular target for TNBC.
EPHA2 Blockade Overcomes Acquired Resistance to EGFR Kinase Inhibitors in Lung Cancer.[Pubmed:26744526]
Cancer Res. 2016 Jan 15;76(2):305-18.
Despite the success of treating EGFR-mutant lung cancer patients with EGFR tyrosine kinase inhibitors (TKI), all patients eventually acquire resistance to these therapies. Although various resistance mechanisms have been described, there are currently no FDA-approved therapies that target alternative mechanisms to treat lung tumors with acquired resistance to first-line EGFR TKI agents. Here we found that EPHA2 is overexpressed in EGFR TKI-resistant tumor cells. Loss of EPHA2 reduced the viability of erlotinib-resistant tumor cells harboring EGFR(T790M) mutations in vitro and inhibited tumor growth and progression in an inducible EGFR(L858R+T790M)-mutant lung cancer model in vivo. Targeting EPHA2 in erlotinib-resistant cells decreased S6K1-mediated phosphorylation of cell death agonist BAD, resulting in reduced tumor cell proliferation and increased apoptosis. Furthermore, pharmacologic inhibition of EPHA2 by the small-molecule inhibitor ALW-II-41-27 decreased both survival and proliferation of erlotinib-resistant tumor cells and inhibited tumor growth in vivo. ALW-II-41-27 was also effective in decreasing viability of cells with acquired resistance to the third-generation EGFR TKI AZD9291. Collectively, these data define a role for EPHA2 in the maintenance of cell survival of TKI-resistant, EGFR-mutant lung cancer and indicate that EPHA2 may serve as a useful therapeutic target in TKI-resistant tumors.
Identification of Novel Small Molecule Inhibitors of Oncogenic RET Kinase.[Pubmed:26046350]
PLoS One. 2015 Jun 5;10(6):e0128364.
Oncogenic mutation of the RET receptor tyrosine kinase is observed in several human malignancies. Here, we describe three novel type II RET tyrosine kinase inhibitors (TKI), ALW-II-41-27, XMD15-44 and HG-6-63-01, that inhibit the cellular activity of oncogenic RET mutants at two digit nanomolar concentration. These three compounds shared a 3-trifluoromethyl-4-methylpiperazinephenyl pharmacophore that stabilizes the 'DFG-out' inactive conformation of RET activation loop. They blocked RET-mediated signaling and proliferation with an IC50 in the nM range in fibroblasts transformed by the RET/C634R and RET/M918T oncogenes. They also inhibited autophosphorylation of several additional oncogenic RET-derived point mutants and chimeric oncogenes. At a concentration of 10 nM, ALW-II-41-27, XMD15-44 and HG-6-63-01 inhibited RET kinase and signaling in human thyroid cancer cell lines carrying oncogenic RET alleles; they also inhibited proliferation of cancer, but not non-tumoral Nthy-ori-3-1, thyroid cells, with an IC50 in the nM range. The three compounds were capable of inhibiting the 'gatekeeper' V804M mutant which confers substantial resistance to established RET inhibitors. In conclusion, we have identified a type II TKI scaffold, shared by ALW-II-41-27, XMD15-44 and HG-6-63-01, that may be used as novel lead for the development of novel agents for the treatment of cancers harboring oncogenic activation of RET.


