Tocrifluor T1117Novel fluorescent cannabinoid ligand; displays GPR55 binding activity CAS# 1186195-59-4 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Regorafenib
Catalog No.:BCC3646
CAS No.:755037-03-7
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1186195-59-4 | SDF | Download SDF |
| PubChem ID | 57369426 | Appearance | Powder |
| Formula | C56H53Cl2N7O5 | M.Wt | 974.97 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
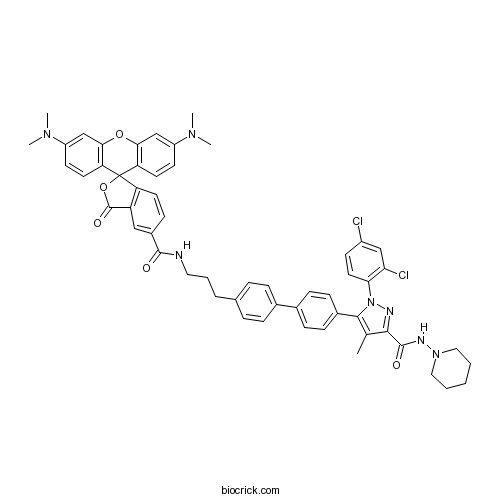
| Chemical Name | 5-[4-[4-[3-[[3',6'-bis(dimethylamino)-3-oxospiro[2-benzofuran-1,9'-xanthene]-5-carbonyl]amino]propyl]phenyl]phenyl]-1-(2,4-dichlorophenyl)-4-methyl-N-piperidin-1-ylpyrazole-3-carboxamide | ||
| SMILES | CC1=C(N(N=C1C(=O)NN2CCCCC2)C3=C(C=C(C=C3)Cl)Cl)C4=CC=C(C=C4)C5=CC=C(C=C5)CCCNC(=O)C6=CC7=C(C=C6)C8(C9=C(C=C(C=C9)N(C)C)OC1=C8C=CC(=C1)N(C)C)OC7=O | ||
| Standard InChIKey | RNAPRXIZPJDYPH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C56H53Cl2N7O5/c1-34-51(54(67)61-64-28-7-6-8-29-64)60-65(48-26-20-40(57)31-47(48)58)52(34)38-17-15-37(16-18-38)36-13-11-35(12-14-36)10-9-27-59-53(66)39-19-23-44-43(30-39)55(68)70-56(44)45-24-21-41(62(2)3)32-49(45)69-50-33-42(63(4)5)22-25-46(50)56/h11-26,30-33H,6-10,27-29H2,1-5H3,(H,59,66)(H,61,67) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fluorescent form of AM 251. AM 251 conjugated with 5-carboxytetramethylrhodamine (5-TAMRA) that fluoresces at 543 nm excitation (590 nm emission). Displays GPR55 binding activity. |

Tocrifluor T1117 Dilution Calculator

Tocrifluor T1117 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0257 mL | 5.1284 mL | 10.2567 mL | 20.5135 mL | 25.6418 mL |
| 5 mM | 0.2051 mL | 1.0257 mL | 2.0513 mL | 4.1027 mL | 5.1284 mL |
| 10 mM | 0.1026 mL | 0.5128 mL | 1.0257 mL | 2.0513 mL | 2.5642 mL |
| 50 mM | 0.0205 mL | 0.1026 mL | 0.2051 mL | 0.4103 mL | 0.5128 mL |
| 100 mM | 0.0103 mL | 0.0513 mL | 0.1026 mL | 0.2051 mL | 0.2564 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BU 226 hydrochloride
Catalog No.:BCC6936
CAS No.:1186195-56-1
- Vitexdoin A
Catalog No.:BCN4089
CAS No.:1186021-77-1
- VD3-D6
Catalog No.:BCC4076
CAS No.:118584-54-6
- NVP-BVU972
Catalog No.:BCC3828
CAS No.:1185763-69-2
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
- Floribundone 1
Catalog No.:BCN4726
CAS No.:118555-84-3
- Phaseoloidin
Catalog No.:BCN8451
CAS No.:118555-82-1
- Boc-Orn(2-Cl-Z)-OH
Catalog No.:BCC3428
CAS No.:118554-00-0
- H-Orn(2-Cl-Z)-OH
Catalog No.:BCC3002
CAS No.:118553-99-4
- Baohuoside V
Catalog No.:BCN2887
CAS No.:118544-18-6
- Icaritin
Catalog No.:BCN5352
CAS No.:118525-40-9
- Sagittatoside C
Catalog No.:BCN3059
CAS No.:118525-37-4
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- NPE-caged-proton
Catalog No.:BCC7698
CAS No.:1186195-63-0
- ALW-II-41-27
Catalog No.:BCC1350
CAS No.:1186206-79-0
- 4SC-202
Catalog No.:BCC5359
CAS No.:1186222-89-8
- Epivogeloside
Catalog No.:BCN6060
CAS No.:118627-52-4
- TMN 355
Catalog No.:BCC6121
CAS No.:1186372-20-2
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- 11-Hydroxycodaphniphylline
Catalog No.:BCN6061
CAS No.:1186496-68-3
- H-Cys(Me)-OH
Catalog No.:BCC2908
CAS No.:1187-84-4
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- Cuniloside B
Catalog No.:BCN6062
CAS No.:1187303-40-7
- Trametinib DMSO solvate
Catalog No.:BCC2013
CAS No.:1187431-43-1
Beyond radio-displacement techniques for identification of CB1 ligands: the first application of a fluorescence-quenching assay.[Pubmed:24441508]
Sci Rep. 2014 Jan 20;4:3757.
Cannabinoid type 1 Receptor (CB1) belongs to the GPCR family and it has been targeted, so far, for the discovery of drugs aimed at the treatment of neuropathic pain, nausea, vomit, and food intake disorders. Here, we present the development of the first fluorescent assay enabling the measurement of kinetic binding constants for CB1 orthosteric ligands. The assay is based on the use of T1117, a fluorescent analogue of AM251. We prove that T1117 binds endogenous and recombinant CB1 receptors with nanomolar affinity. Moreover, T1117 binding to CB1 is sensitive to the allosteric ligand ORG27569 and thus it is applicable to the discovery of new allosteric drugs. The herein presented assay constitutes a sustainable valid alternative to the expensive and environmental impacting radiodisplacement techniques and paves the way for an easy, fast and cheap high-throughput drug screening toward CB1 for identification of new orthosteric and allosteric modulators.
Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses.[Pubmed:23472002]
Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):5193-8.
G protein-coupled receptor (GPR) 55 is sensitive to certain cannabinoids, it is expressed in the brain and, in cell cultures, it triggers mobilization of intracellular Ca(2+). However, the adaptive neurobiological significance of GPR55 remains unknown. Here, we use acute hippocampal slices and combine two-photon excitation Ca(2+) imaging in presynaptic axonal boutons with optical quantal analysis in postsynaptic dendritic spines to find that GPR55 activation transiently increases release probability at individual CA3-CA1 synapses. The underlying mechanism involves Ca(2+) release from presynaptic Ca(2+) stores, whereas postsynaptic stores (activated by spot-uncaging of inositol 1,4,5-trisphosphate) remain unaffected by GPR55 agonists. These effects are abolished by genetic deletion of GPR55 or by the GPR55 antagonist cannabidiol, a constituent of Cannabis sativa. GPR55 shows colocalization with synaptic vesicle protein vesicular glutamate transporter 1 in stratum radiatum. Short-term potentiation of CA3-CA1 transmission after a short train of stimuli reveals a presynaptic, Ca(2+) store-dependent component sensitive to cannabidiol. The underlying cascade involves synthesis of phospholipids, likely in the presynaptic cell, but not the endocannabinoids 2-arachidonoylglycerol or anandamide. Our results thus unveil a signaling role for GPR55 in synaptic circuits of the brain.
Fluorescent ligand binding reveals heterogeneous distribution of adrenoceptors and 'cannabinoid-like' receptors in small arteries.[Pubmed:20136833]
Br J Pharmacol. 2010 Feb;159(4):787-96.
BACKGROUND AND PURPOSE: Pharmacological analysis of synergism or functional antagonism between different receptors commonly assumes that interacting receptors are located in the same cells. We have now investigated the distribution of alpha-adrenoceptors, beta-adrenoceptors and cannabinoid-like (GPR55) receptors in the mouse arteries. EXPERIMENTAL APPROACH: Fluorescence intensity from vascular tissue incubated with fluorescent ligands (alpha(1)-adrenoceptor ligand, BODIPY-FL-prazosin, QAPB; beta-adrenoceptor ligand, TMR-CGP12177; fluorescent angiotensin II; a novel diarylpyrazole cannabinoid ligand (Tocrifluor 1117, T1117) was measured with confocal microscopy. Small mesenteric and tail arteries of wild-type and alpha(1B/D)-adrenoceptor-KO mice were used. KEY RESULTS: T1117, a fluorescent form of the cannabinoid CB(1) receptor antagonist AM251, was a ligand for GPR55, with low affinity for CB(1) receptors. In mesenteric arterial smooth muscle cells, alpha(1A)-adrenoceptors were predominantly located in different cells from those with beta-adrenoceptors, angiotensin receptors or cannabinoid-like (GPR55) receptors. Cells with beta-adrenoceptors predominated at arterial branches. Endothelial cells expressed beta-adrenoceptors, alpha-adrenoceptors and cannabinoid-like receptors. Only endothelial alpha-adrenoceptors appeared in clusters. Adventitia was a rich source of G protein-coupled receptors (GPCRs), particularly fibroblasts and nerve tracts, where Schwann cells bound alpha-adrenoceptor, beta-adrenoceptor and CB-receptor ligands, with a mix of separate receptor locations and co-localization. CONCLUSIONS AND IMPLICATIONS: Within each cell type, each GPCR had a distinctive heterogeneous distribution with limited co-localization, providing a guide to the possibilities for functional synergism, and suggesting a new paradigm for synergism in which interactions may be either between cells or involve converging intracellular signalling processes.


