JDTicOpioid antagonist CAS# 361444-66-8 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 361444-66-8 | SDF | Download SDF |
| PubChem ID | 9956146 | Appearance | Powder |
| Formula | C28H39N3O3 | M.Wt | 465.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
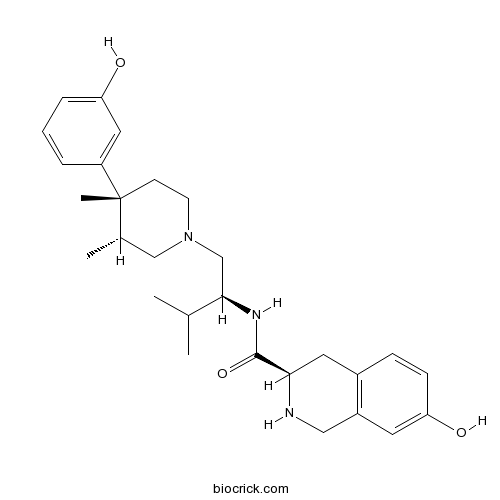
| Chemical Name | (3R)-7-hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide | ||
| SMILES | CC1CN(CCC1(C)C2=CC(=CC=C2)O)CC(C(C)C)NC(=O)C3CC4=C(CN3)C=C(C=C4)O | ||
| Standard InChIKey | ZLVXBBHTMQJRSX-VMGNSXQWSA-N | ||
| Standard InChI | InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25+,26+,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

JDTic Dilution Calculator

JDTic Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1476 mL | 10.7381 mL | 21.4763 mL | 42.9526 mL | 53.6907 mL |
| 5 mM | 0.4295 mL | 2.1476 mL | 4.2953 mL | 8.5905 mL | 10.7381 mL |
| 10 mM | 0.2148 mL | 1.0738 mL | 2.1476 mL | 4.2953 mL | 5.3691 mL |
| 50 mM | 0.043 mL | 0.2148 mL | 0.4295 mL | 0.8591 mL | 1.0738 mL |
| 100 mM | 0.0215 mL | 0.1074 mL | 0.2148 mL | 0.4295 mL | 0.5369 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ki = 0.3 nM
JDTic is a selective opioid Kappa receptor antagonist.
At least three opioid receptor subtypes, μ, δ, and κ, are responsible for the modulation of a diverse array of biological events from nociception to immune regulation. The baisi of studying this complex receptor system is for the identification of both agonists and antagonists with high degree of receptor subtype selectivity.
In vitro: JDTic demonstrated high affinity for the κ receptor in the binding assay and highly potent and selective κ antagonism in the [35S]GTP-γ-S assay. JDTic showed a 16-fold improvement in its κ receptor Ki value in the functional assay relative to the binding assay. In the [35S]GTP-γ-S functional assay, JDTic demonstrated a 3.4-fold increase in κ antagonist potency relative to the functional assay utilizing guinea pig membranes [1].
In vivo: JDTic was found to be able to dose-dependently block acute nicotineinduced antinociception in the tail-flick but not the hotplate test and did not attenuate morphine's antinociceptive effect significantly in either test. Moreover, JDTic failed to block the expression of nicotine reward as measured by the conditioned place preference model. In contrast, JDTic attenuated the expression of both physical and affective nicotine withdrawal signs in mice[2].
Clinical trial: N/A
References:
[1] Thomas JB,Atkinson RN,Rothman RB,Fix SE,Mascarella SW,Vinson NA,Xu H,Dersch CM,Lu Y,Cantrell BE,Zimmerman DM,Carroll FI. Identification of the first trans-(3R,4R)- dimethyl-4-(3-hydroxyphenyl)piperidine derivative to possess highly potent and selective opioid kappa receptor antagonist activity. J Med Chem.2001 Aug 16;44(17):2687-90.
[2] Jackson KJ,Carroll FI,Negus SS,Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl).2010 Jun;210(2):285-94.
- Saxagliptin
Catalog No.:BCC3934
CAS No.:361442-04-8
- 3,4-Secolupa-4(23),20(29)-diene-3,28-dioic acid
Catalog No.:BCN7243
CAS No.:36138-41-7
- RBC8
Catalog No.:BCC5569
CAS No.:361185-42-4
- Propranolol glycol
Catalog No.:BCC6817
CAS No.:36112-95-5
- Sodium cholate
Catalog No.:BCN6981
CAS No.:361-09-1
- B-HT 920
Catalog No.:BCC1417
CAS No.:36085-73-1
- B-HT 933 dihydrochloride
Catalog No.:BCC7474
CAS No.:36067-72-8
- Octahydrocurcumin
Catalog No.:BCN2725
CAS No.:36062-07-4
- Hexahydrocurcumin
Catalog No.:BCN4641
CAS No.:36062-05-2
- Tetrahydrocurcumin
Catalog No.:BCN2724
CAS No.:36062-04-1
- Dihydrosanguinarine
Catalog No.:BCN3713
CAS No.:3606-45-9
- Alpinetin
Catalog No.:BCN5315
CAS No.:36052-37-6
- D-(+)-Fucose
Catalog No.:BCN6432
CAS No.:3615-37-0
- alpha-L-Rhamnose
Catalog No.:BCN2592
CAS No.:3615-41-6
- Phytin
Catalog No.:BCN1285
CAS No.:3615-82-5
- Mullilam diol
Catalog No.:BCN5316
CAS No.:36150-04-6
- Saxalin
Catalog No.:BCC8357
CAS No.:36150-06-8
- Dehydroleucodine
Catalog No.:BCN6897
CAS No.:36150-07-9
- Kobusin
Catalog No.:BCN7563
CAS No.:36150-23-9
- Blumenol B
Catalog No.:BCN5317
CAS No.:36151-01-6
- 3-Acetyl-2,5-dichlorothiophene
Catalog No.:BCC8602
CAS No.:36157-40-1
- 3'-O-Methylorobol
Catalog No.:BCN5318
CAS No.:36190-95-1
- Genistein 7,4'-di-O-beta-D-glucopyranoside
Catalog No.:BCN7835
CAS No.:36190-98-4
- 2-Methoxyestradiol (2-MeOE2)
Catalog No.:BCC2228
CAS No.:362-07-2
Pharmacodynamic Relationships between Duration of Action of JDTic-like Kappa-Opioid Receptor Antagonists and Their Brain and Plasma Pharmacokinetics in Rats.[Pubmed:27700049]
ACS Chem Neurosci. 2016 Dec 21;7(12):1737-1745.
JDTic is a potent and selective kappa-opioid receptor (KOR) antagonist that reverses U50,488-induced diuresis in rats. It partitions into brain with a duration of action lasting for weeks. In a search for KOR antagonists that do not accumulate in the brain, we compared single doses of five methylated JDTic analogs (RTI-97, -194, -212, -240, and -241) for reversal of U50,488 diuresis and pharmacokinetic (PK) properties. All six compounds showed potent and selective KOR antagonism in a [(35)S]GTPgammaS binding assay. Plasma half-lives ranged from 24 to 41 h and brain half-lives from 24 to 76 h. JDTic and RTI-194 showed increasing brain to plasma ratios over time, indicating increasing partitioning into brain and a longer duration of action for reversal of diuresis than did RTI-97. RTI-240 did not show significant brain accumulation. RTI-212 showed no substantive difference between brain and plasma levels and was inactive against diuresis. RTI-241, with a lower brain to plasma ratio than JDTic and RTI-194, formed JDTic as a metabolite, which still reduced diuresis after 9 weeks. The fact that the duration of action was correlated with the brain to blood plasma ratios and area under the concentration-time curves suggests that PK properties could help to predict safety and acceptable duration of action for KOR antagonists.
Design, synthesis, and pharmacological evaluation of JDTic analogs to examine the significance of replacement of the 3-hydroxyphenyl group with pyridine or thiophene bioisosteres.[Pubmed:27364611]
Bioorg Med Chem. 2016 Aug 15;24(16):3842-8.
The potent and selective KOR antagonist JDTic was derived from the N-substituted trans-3,4-dimethyl-4-(3-hydroxyphenyl)piperidine class of pure opioid antagonists. In previous studies we reported that compounds that did not have a hydroxyl on the 3-hydroxyphenyl group and did not have methyl groups at the 3- and 4-position of the piperidine ring were still potent and selective KOR antagonists. In this study we report JDTic analogs 2, 3a-b, 4a-b, and 5, where the 3-hydroxyphenyl ring has been replaced by a 2-, 3-, or 4-pyridyl or 3-thienyl group and do not have the 3-methyl or 3,4-dimethyl groups, remain potent and selective KOR antagonists. Of these, (3R)-7-hydroxy-N-(1S)-2-methyl-[4-methyl-4-pyridine-3-yl-carboxamide (3b) had the best overall binding potency and selectivity in a [(35)S]GTPgammaS functional assay, with a Ke=0.18nM at the KOR and 273- and 16,700-fold selectivity for the KOR relative to the MOR and DOR, respectively. Calculated physiochemical properties for 3b suggest that it will cross the blood-brain barrier.
N-[(18)F]-FluoropropylJDTic for kappa-opioid receptor PET imaging: Radiosynthesis, pre-clinical evaluation, and metabolic investigation in comparison with parent JDTic.[Pubmed:27821345]
Nucl Med Biol. 2017 Jan;44:50-61.
INTRODUCTION: To image kappa opioid receptor (KOR) for preclinical studies, N-fluoropropylJDTic 9 derived from the best-established KOR antagonist JDTic, was labeled with fluorine-18. METHODS: Radiosynthesis of [(18)F]9 was achieved according to an automated two-step procedure from [(18)F]-fluoride. Peripheral and cerebral distributions were determined by ex vivo experiments and by PET imaging in mouse. Radiometabolism studies were performed both in vivo in mice and in vitro in mouse and human liver microsomes. Identification of the major metabolic fragmentations was carried out by UPLC-MS analysis of enzymatic cleavage of non-radioactive ligand 9. Microsomal metabolic degradation of parent JDTic was also achieved for comparison. RESULTS: The radiotracer [(18)F]9 was produced after 140+/-5min total synthesis time (2.2+/-0.4% not decay corrected radiochemical yield) with a specific activity of 41-89GBq/mumol (1.1-2.4Ci/mumol). Peripheral and regional brain distributions of [(18)F]9 were consistent with known KOR locations but no significant specific binding in brain was shown. [(18)F]9 presented a typical hepatobiliary and renal elimination, and was rapidly metabolized. The in vivo and in vitro radiometabolic profiles of [(18)F]9 were similar. Piperidine 12 was identified as the major metabolic fragment of the non-radioactive ligand 9. JDTic 7 was found to be much more stable than 9. CONCLUSION: Although the newly proposed radioligand [(18)F]9 was concluded to be not suitable for KOR PET imaging due to the formation of brain penetrating radiometabolites, our findings highlight the metabolic stability of JDTic and may help in the design of novel JDTic derivatives for in vivo applications.
Design, synthesis, and pharmacological evaluation of JDTic analogs to examine the significance of the 3- and 4-methyl substituents.[Pubmed:26342544]
Bioorg Med Chem. 2015 Oct 1;23(19):6379-88.
The design and discovery of JDTic as a potent and selective kappa opioid receptor antagonist used the N-substituted trans-3,4-dimethyl-4-(3-hydroxyphenyl)piperidine pharmacophore as the lead structure. In order to determine if the 3-methyl or 4-methyl groups were necessary in JDTic and JDTic analogs for antagonistic activity, compounds 4a-c, and 4d-f which have either the 3-methyl or both the 3- and 4-methyl groups removed, respectively, from JDTic and analogs were synthesized and evaluated for their in vitro opioid receptor antagonist activities using a [(35)S]GTPgammaS binding assay. Other ADME properties were also assessed for selected compounds. These studies demonstrated that neither the 3-methyl or 3,4-dimethyl groups present in JDTic and analogs are required to produce potent and selective kappa opioid receptor antagonists.


