pep2-SVKIPeptide inhibitor of GluR2 subunit binding to GRIP, ABP and PICK1. Increases AMPA current amplitude CAS# 328944-75-8 |
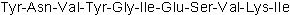
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- CGS 21680 HCl
Catalog No.:BCC4316
CAS No.:124431-80-7
- Valsartan
Catalog No.:BCC5017
CAS No.:137862-53-4
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- Ticlopidine HCl
Catalog No.:BCC4973
CAS No.:53885-35-1
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure
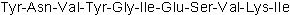
3D structure
| Cas No. | 328944-75-8 | SDF | Download SDF |
| PubChem ID | 90479807 | Appearance | Powder |
| Formula | C60H93N13O18 | M.Wt | 1284.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | YNVYGIESVKI | ||
| Chemical Name | (2S,3S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-4-oxobutanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]acetyl]amino]-3-methylpentanoyl]amino]-4-carboxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-methylbutanoyl]amino]hexanoyl]amino]-3-methylpentanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CCC(=O)O)C(=O)NC(CO)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NC(C(C)CC)C(=O)O)NC(=O)CNC(=O)C(CC1=CC=C(C=C1)O)NC(=O)C(C(C)C)NC(=O)C(CC(=O)N)NC(=O)C(CC2=CC=C(C=C2)O)N | ||
| Standard InChIKey | YWTNLHXVGSIPEM-YDSVATBTSA-N | ||
| Standard InChI | InChI=1S/C60H93N13O18/c1-9-32(7)49(59(89)66-40(22-23-46(79)80)53(83)69-43(29-74)56(86)72-47(30(3)4)57(87)65-39(13-11-12-24-61)54(84)73-50(60(90)91)33(8)10-2)70-45(78)28-64-52(82)41(26-35-16-20-37(76)21-17-35)68-58(88)48(31(5)6)71-55(85)42(27-44(63)77)67-51(81)38(62)25-34-14-18-36(75)19-15-34/h14-21,30-33,38-43,47-50,74-76H,9-13,22-29,61-62H2,1-8H3,(H2,63,77)(H,64,82)(H,65,87)(H,66,89)(H,67,81)(H,68,88)(H,69,83)(H,70,78)(H,71,85)(H,72,86)(H,73,84)(H,79,80)(H,90,91)/t32-,33-,38-,39-,40-,41-,42-,43-,47-,48-,49-,50-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor peptide corresponding to last 10 amino acids of the C-terminus of the GluR2 AMPA receptor subunit. Disrupts binding of GluR2 (at the C-terminal PDZ site) with glutamate receptor interacting protein (GRIP), AMPA receptor binding protein (ABP) and protein interacting with C kinase (PICK1). Increases amplitude of AMPA receptor-mediated currents and blocks long-term depression (LTD). Control peptide available. |

pep2-SVKI Dilution Calculator

pep2-SVKI Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tildipirosin
Catalog No.:BCC5478
CAS No.:328898-40-4
- De-O-methyllasiodiplodin
Catalog No.:BCN7187
CAS No.:32885-82-8
- Lasiodiplodin
Catalog No.:BCN4770
CAS No.:32885-81-7
- Cajanin
Catalog No.:BCN5249
CAS No.:32884-36-9
- Benzylamine hydrochloride
Catalog No.:BCN6908
CAS No.:3287-99-8
- AG-14361
Catalog No.:BCC2209
CAS No.:328543-09-5
- GlyH-101
Catalog No.:BCC4104
CAS No.:328541-79-3
- Lannaconitine
Catalog No.:BCN2504
CAS No.:32854-75-4
- (H-Cys-OMe)2.2HCl
Catalog No.:BCC2916
CAS No.:32854-09-4
- Coniferyl alcohol
Catalog No.:BCN4651
CAS No.:32811-40-8
- Phortress
Catalog No.:BCC3901
CAS No.:328087-38-3
- Ceranib 1
Catalog No.:BCC6186
CAS No.:328076-61-5
- C646
Catalog No.:BCC4546
CAS No.:328968-36-1
- 4E1RCat
Catalog No.:BCC5338
CAS No.:328998-25-0
- Norepinephrine hydrochloride
Catalog No.:BCC5133
CAS No.:329-56-6
- DL-Adrenaline
Catalog No.:BCC4318
CAS No.:329-65-7
- PMSF
Catalog No.:BCC1229
CAS No.:329-98-6
- Withanolide A
Catalog No.:BCN8010
CAS No.:32911-62-9
- SANT-2
Catalog No.:BCC3937
CAS No.:329196-48-7
- Quetiapine hydroxy impurity
Catalog No.:BCN5340
CAS No.:329216-67-3
- Boc-His(Trt)-OH
Catalog No.:BCC3403
CAS No.:32926-43-5
- Sanggenol L
Catalog No.:BCN3692
CAS No.:329319-20-2
- 7-O-Methylaloeresin A
Catalog No.:BCN2849
CAS No.:329361-25-3
- FPR A14
Catalog No.:BCC7498
CAS No.:329691-12-5
Nicotinic receptors modulate the function of presynaptic AMPA receptors on glutamatergic nerve terminals in the trigeminal caudal nucleus.[Pubmed:26277383]
Neurochem Int. 2015 Nov;90:166-72.
In this study, we demonstrate the existence on trigeminal caudal nucleus (TCN) glutamatergic terminals of alpha4beta2 nicotinic receptors (nAChRs) capable of enhancing the terminals' spontaneous release of [(3)H]d-aspartate ([(3)H]D-Asp). In rat TCN synaptosomes, spontaneous [(3)H]D-Asp release was increased by nicotine and the nicotinic receptor agonists (+/-)epibatidine and RJR2403. The increase was potentiated by the positive allosteric modulator of nAChRs LY2087101, inhibited by the nicotinic antagonists mecamylamine (MEC) and dihydro-beta-erythroidine hydrobromide (DHbetaE), and unaffected by alpha-bungarotoxin (alpha-BgTx) and methyllycaconitine (MLA). Evidence of functional interaction was observed between the alpha4beta2 nAChRs and cyclothiazide-sensitive, alfa-amino-3-hydroxy-5-methyl-4-isoxazolone propionate (AMPA) receptors co-localized on the TCN synaptosomes. Brief pre-exposure of synaptosomes to 30 muM nicotine or 10 muM RJR2403 abolished the AMPA (100 muM) -induced potentiation of [K(+)]e-evoked [(3)H]D-Asp release, an effect that seems to be caused by nicotine-induced increases in the internalization of AMPA receptors. Indeed, the effects of nicotine-pretreatment were not seen in synaptosomes containing pre-entrapped pep2-SVKI, a peptide known to compete for the binding of GluA2 subunit to scaffolding proteins involved in AMPA endocytosis, while entrapment of pep2-SVKE, an inactive peptide used as negative control, was inefficacious. These findings show that nicotine can negatively modulate the function of AMPA receptors present on glutamatergic nerve terminals in the rat TCN. Dynamic control of AMPA receptors by the nicotinic cholinergic system has been observed under other experimental conditions, and it can contribute to the control of synaptic plasticity such as long-term depression and potentiation. Nicotine's ability to reduce the functionality of presynaptic AMPA receptors could contribute to its analgesic effects by diminishing glutamatergic transmission from the primary afferent terminals that convey nociceptive input to TCN.
In vitro exposure to nicotine induces endocytosis of presynaptic AMPA receptors modulating dopamine release in rat nucleus accumbens nerve terminals.[Pubmed:22771975]
Neuropharmacology. 2012 Oct;63(5):916-26.
Here we provide functional and immunocytochemical evidence supporting the presence on Nucleus Accumbens (NAc) dopaminergic terminals of cyclothiazide-sensitive, alfa-amino-3-hydroxy-5-methyl-4-isoxazolone propionate (AMPA) receptors, which activation causes Ca(2)(+)-dependent [(3)H]dopamine ([(3)H]DA) exocytosis. These AMPA receptors cross-talk with co-localized nicotinic receptors (nAChRs), as suggested by the finding that in vitro short-term pre-exposure of synaptosomes to 30 muM nicotine caused a significant reduction of both the 30 muM nicotine and the 100 muM AMPA-evoked [(3)H]DA overflow. Entrapping pep2-SVKI, a peptide known to compete for the binding of GluA2 subunit to scaffolding proteins involved in AMPA receptor endocytosis, in NAC synaptosomes prevented the nicotine-induced reduction of AMPA-mediated [(3)H]DA exocytosis, while pep2-SVKE, used as negative control, was inefficacious. Immunocytochemical studies showed that a significant percentage of NAc terminals were dopaminergic and that most of these terminals also posses GluA2 receptor subunits. Western blot analysis of GluA2 immunoreactivity showed that presynaptic GluA2 proteins in NAc terminals were reduced in nicotine-pretreated synaptosomes when compared to the control. The nACh-AMPA receptor-receptor interaction was not limited to dopaminergic terminals since nicotine pre-exposure also affected the presynaptic AMPA receptors controlling hippocampal noradrenaline release, but not the presynaptic AMPA receptors controlling GABA and acetylcholine release. These observations could be relevant to the comprehension of the molecular mechanisms at the basis of nicotine rewarding.
Co-induction of LTP and LTD and its regulation by protein kinases and phosphatases.[Pubmed:20457859]
J Neurophysiol. 2010 May;103(5):2737-46.
The cellular properties of long-term potentiation (LTP) following pairing of pre- and postsynaptic activity were examined at a known glutamatergic synapse in the leech, specifically between the pressure (P) mechanosensory and anterior pagoda (AP) neurons. Stimulation of the presynaptic P cell (25 Hz) concurrent with a 2 nA depolarization of the postsynaptic AP cell significantly potentiated the P-to-AP excitatory postsynaptic potential (EPSP) in an N-methyl-d-aspartate receptor (NMDAR)-dependent manner based on inhibitory effects of the NMDAR antagonist MK801 and inhibition of the NMDAR glycine binding site by 7-chlorokynurenic acid. LTP was blocked by injection of bis-(o-aminophenoxy)-N,N,N',N'-tetraacetic acid (BAPTA) into the postsynaptic (AP) cell, indicating a requirement for postsynaptic elevation of intracellular Ca(2+). Autocamtide-2-related inhibitory peptide (AIP), a specific inhibitor of Ca(2+)/calmodulin-dependent kinase II (CaMKII), and Rp-cAMP, an inhibitor of protein kinase A (PKA), also blocked pairing-induced potentiation, indicating a requirement for activation of CaMKII and PKA. Interestingly, application of AIP during pairing resulted in significantly depressed synaptic transmission. Co-application of AIP with the protein phosphatase inhibitor okadaic acid restored synaptic transmission to baseline levels, suggesting an interaction between CaMKII and protein phosphatases during induction of activity-dependent synaptic plasticity. When postsynaptic activity preceded presynaptic activity, NMDAR-dependent long-term depression (LTD) was observed that was blocked by okadaic acid. Postsynaptic injection of botulinum toxin blocked P-to-AP potentiation while postsynaptic injection of pep2-SVKI, an inhibitor of AMPA receptor endocytosis, inhibited LTD, supporting the hypothesis that glutamate receptor trafficking contributes to both LTP and LTD at the P-to-AP synapse in the leech.
PDZ protein mediated activity-dependent LTP/LTD developmental switch at rat retinocollicular synapses.[Pubmed:20457829]
Am J Physiol Cell Physiol. 2010 Jun;298(6):C1572-82.
The insertion of amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors into the plasma membrane and removal via internalization are essential for regulating synaptic strength, which underlies the basic mechanism of learning and memory. The retinocollicular pathway undergoes synaptic refinement during development and shows a wide variety of long-term synaptic changes; however, still little is known about its underlying molecular regulation. Here we report a rapid developmental long-term potentiation (LTP)/long-term depression (LTD) switch and its intracellular mechanism at the rat retinocollicular pathway from postnatal day 5 (P5) to P14. Before P9, neurons always exhibited LTP, whereas LTD was observed only after P10. Blockade of GluR2/3-glutamate receptor-interacting protein (GRIP)/AMPA-receptor-binding protein (ABP)/protein interacting with C kinase 1 (PICK1) interactions with pep2-SVKI could sustain the LTP after P10. This suggests that the LTP/LTD switch relied on PDZ protein activities. Selective interruption of GluR2/3-PICK1 binding by pep2-EVKI blocked the long-lasting effects of both LTP and LTD, suggesting a role for PICK1 in the maintenance of long-term synaptic plasticity. Interestingly, synaptic expression of GRIP increased more than twofold from P7 to P11, whereas ABP and PICK1 expression levels remained stable. Blockade of spontaneous retinal input suppressed this increase and abolished the LTP/LTD switch. These results suggest that the increased GRIP synaptic expression may be a key regulatory factor in mediating the activity-dependent developmental LTP/LTD switch, whereas PICK1 may be required for both LTP and LTD to maintain their long-term effects.
CNQX and AMPA inhibit electrical synaptic transmission: a potential interaction between electrical and glutamatergic synapses.[Pubmed:18601913]
Brain Res. 2008 Sep 4;1228:43-57.
Electrical synapses play an important role in signaling between neurons and the synaptic connections between many neurons possess both electrical and chemical components. Although modulation of electrical synapses is frequently observed, the cellular processes that mediate such changes have not been studied as thoroughly as plasticity in chemical synapses. In the leech (Hirudo sp), the competitive AMPA receptor antagonist CNQX inhibited transmission at the rectifying electrical synapse of a mixed glutamatergic/electrical synaptic connection. This CNQX-mediated inhibition of the electrical synapse was blocked by concanavalin A (Con A) and dynamin inhibitory peptide (DIP), both of which are known to inhibit endocytosis of neurotransmitter receptors. CNQX-mediated inhibition was also blocked by pep2-SVKI (SVKI), a synthetic peptide that prevents internalization of AMPA-type glutamate receptor. AMPA itself also inhibited electrical synaptic transmission and this AMPA-mediated inhibition was partially blocked by Con A, DIP and SVKI. Low frequency stimulation induced long-term depression (LTD) in both the electrical and glutamatergic components of these synapses and this LTD was blocked by SVKI. GYKI 52466, a selective non-competitive antagonist of AMPA receptors, did not affect the electrical EPSP, although it did block the glutamatergic component of these synapses. CNQX did not affect non-rectifying electrical synapses in two different pairs of neurons. These results suggest an interaction between AMPA-type glutamate receptors and the gap junction proteins that mediate electrical synaptic transmission. This putative interaction between glutamate receptors and gap junction proteins represents a novel mechanism for regulating the strength of synaptic transmission.
Trafficking of presynaptic AMPA receptors mediating neurotransmitter release: neuronal selectivity and relationships with sensitivity to cyclothiazide.[Pubmed:16242162]
Neuropharmacology. 2006 Mar;50(3):286-96.
Postsynaptic glutamate AMPA receptors (AMPARs) can recycle between plasma membrane and intracellular pools. In contrast, trafficking of presynaptic AMPARs has not been investigated. AMPAR surface expression involves interactions between the GluR2 carboxy tail and various proteins including glutamate receptor-interacting protein (GRIP), AMPA receptor-binding protein (ABP), protein interacting with C kinase 1 (PICK1), N-ethyl-maleimide-sensitive fusion protein (NSF). Here, peptides known to selectively block the above interactions were entrapped into synaptosomes to study the effects on the AMPA-evoked release of [3H]noradrenaline ([3H]NA) and [3H]acetylcholine ([3H]ACh) from rat hippocampal and cortical synaptosomes, respectively. Internalization of pep2-SVKI to prevent GluR2-GRIP/ABP/PICK1 interactions potentiated the AMPA-evoked release of [3H]NA but left unmodified that of [3H]ACh. Similar potentiation was caused by pep2-AVKI, the blocker of GluR2-PICK1 interaction. Conversely, a decrease in the AMPA-evoked release of [3H]NA, but not of [3H]ACh, was caused by pep2m, a selective blocker of the GluR2-NSF interaction. In the presence of pep2-SVKI the presynaptic AMPARs on noradrenergic terminals lost sensitivity to cyclothiazide. AMPARs releasing [3H]ACh, but not those releasing [3H]NA, were sensitive to spermine, suggesting that they are GluR2-lacking AMPARs. To conclude: (i) release-regulating presynaptic AMPARs constitutively cycle in isolated nerve terminals; (ii) the process exhibits neuronal selectivity; (iii) AMPAR trafficking and desensitization may be interrelated.
PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses.[Pubmed:11163273]
Neuron. 2000 Dec;28(3):873-86.
We investigated the role of PDZ proteins (GRIP, ABP, and PICK1) interacting with the C-terminal GluR2 by infusing a ct-GluR2 peptide ("pep2-SVKI") into CA1 pyramidal neurons in hippocampal slices using whole-cell recordings. pep2-SVKI, but not a control or PICK1 selective peptide, caused AMPAR-mediated EPSC amplitude to increase in approximately one-third of control neurons and in most neurons following the prior induction of LTD. pep2-SVKI also blocked LTD; however, this occurred in all neurons. A PKC inhibitor prevented these effects of pep2-SVKI on synaptic transmission and LTD. We propose a model in which the maintenance of LTD involves the binding of AMPARs to PDZ proteins to prevent their reinsertion. We also present evidence that PKC regulates AMPAR reinsertion during dedepression.
Functional roles of protein interactions with AMPA and kainate receptors.[Pubmed:12941441]
Neurosci Res. 2003 Sep;47(1):3-15.
The glutamate receptor subtypes AMPA and kainate are involved in synaptic transmission and synaptic plasticity in the CNS. Recently there has been considerable interest in understanding the molecular regulation of these receptors by proteins that directly bind to AMPA and kainate receptor subunits. Amongst the first interaction partners to be discovered were NSF, ABP, GRIP and PICK1, which bind the AMPA receptor subunit GLUA2. We have studied the functional roles of the interactions of these proteins in regulating AMPA receptor-mediated synaptic transmission and synaptic plasticity in the hippocampus. We have also started to investigate the functions of PICK1 and GRIP on kainate receptor-mediated synaptic transmission in this region. In this article we reflect upon this work, which has led to some new ideas about how AMPA and kainate receptors are regulated at synapses.
Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression.[Pubmed:11573007]
Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11725-30.
The interaction of PDZ domain-containing proteins with the C termini of alpha-amino-3-hydroxy-5-methylisoxazolepropionate (AMPA) receptors has been suggested to be important in the regulation of receptor targeting to excitatory synapses. Recent studies have shown that the rapid internalization of AMPA receptors at synapses may mediate, at least in part, the expression of long-term depression (LTD). We have previously shown that phosphorylation of Ser-880 on the AMPA receptor GluR2 subunit differentially regulated the interaction of GluR2 with the PDZ domain-containing proteins GRIP1 and PICK1. Here, we show that induction of LTD in hippocampal slices increases phosphorylation of Ser-880 within the GluR2 C-terminal PDZ ligand, suggesting that the modulation of GluR2 interaction with GRIP1 and PICK1 may regulate AMPA receptor internalization during LTD. Moreover, postsynaptic intracellular perfusion of GluR2 C-terminal peptides that disrupt GluR2 interaction with PICK1 inhibit the expression of hippocampal LTD. These results suggest that the interaction of GluR2 with PICK1 may play a regulatory role in the expression of LTD in the hippocampus.
AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses.[Pubmed:10526335]
Nat Neurosci. 1999 Nov;2(11):972-7.
Silent synapses form between some primary sensory afferents and dorsal horn neurons in the spinal cord. Molecular mechanisms for activation or conversion of silent synapses to conducting synapses are unknown. Serotonin can trigger activation of silent synapses in dorsal horn neurons by recruiting AMPA receptors. AMPA-receptor subunits GluR2 and GluR3 interact via their cytoplasmic C termini with PDZ-domain-containing proteins such as GRIP (glutamate receptor interacting protein), but the functional significance of these interactions is unclear. Here we demonstrate that protein interactions involving the GluR2/3 C terminus are important for serotonin-induced activation of silent synapses in the spinal cord. Furthermore, PKC is a necessary and sufficient trigger for this activation. These results implicate AMPA receptor-PDZ interactions in mechanisms underlying sensory synaptic potentiation and provide insights into the pathogenesis of chronic pain.


