GNF179 MetaboliteMetabolite of GNF179 CAS# 1310455-86-7 |
- Lestaurtinib
Catalog No.:BCC2440
CAS No.:111358-88-4
- GW441756
Catalog No.:BCC5093
CAS No.:504433-23-2
- TLQP 21
Catalog No.:BCC2405
CAS No.:869988-94-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1310455-86-7 | SDF | Download SDF |
| PubChem ID | 57521783 | Appearance | Powder |
| Formula | C14H16FN3 | M.Wt | 245.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
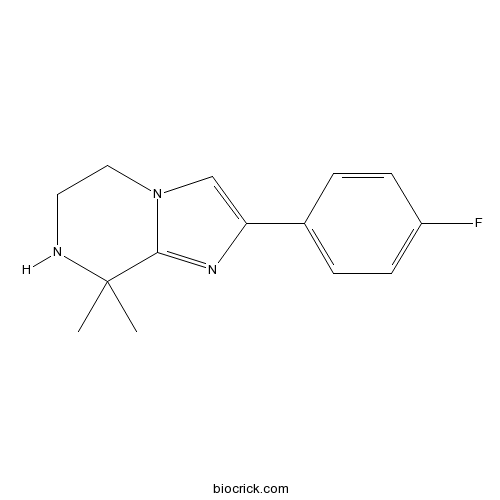
| Chemical Name | 2-(4-fluorophenyl)-8,8-dimethyl-6,7-dihydro-5H-imidazo[1,2-a]pyrazine | ||
| SMILES | CC1(C2=NC(=CN2CCN1)C3=CC=C(C=C3)F)C | ||
| Standard InChIKey | GZVLWGZMELYUPG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H16FN3/c1-14(2)13-17-12(9-18(13)8-7-16-14)10-3-5-11(15)6-4-10/h3-6,9,16H,7-8H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

GNF179 Metabolite Dilution Calculator

GNF179 Metabolite Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0766 mL | 20.3832 mL | 40.7664 mL | 81.5328 mL | 101.916 mL |
| 5 mM | 0.8153 mL | 4.0766 mL | 8.1533 mL | 16.3066 mL | 20.3832 mL |
| 10 mM | 0.4077 mL | 2.0383 mL | 4.0766 mL | 8.1533 mL | 10.1916 mL |
| 50 mM | 0.0815 mL | 0.4077 mL | 0.8153 mL | 1.6307 mL | 2.0383 mL |
| 100 mM | 0.0408 mL | 0.2038 mL | 0.4077 mL | 0.8153 mL | 1.0192 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GNF179 metabolite is the metabolite of GNF179, which is an optimized 8,8-dimethyl IP analog that exhibited the potency(4.8 nM against the multidrug resistant strain W2) in vitro metabolic stability and in vivo oral bioavailability.
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- 8-Epiloganic acid
Catalog No.:BCC8956
CAS No.:82509-41-9
- Meptyldinocap
Catalog No.:BCC5468
CAS No.:131-72-6
- Oxybenzone
Catalog No.:BCC5445
CAS No.:131-57-7
- N-Acetylneuraminic acid
Catalog No.:BCN2204
CAS No.:131-48-6
- Pimpinellin
Catalog No.:BCN6168
CAS No.:131-12-4
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- Sephin1
Catalog No.:BCC3980
CAS No.:13098-73-2
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Miltipolone
Catalog No.:BCN3222
CAS No.:131086-61-8
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- Sodium Demethylcantharidate
Catalog No.:BCN8394
CAS No.:13114-29-9
- NSC 624206
Catalog No.:BCC7988
CAS No.:13116-77-3
- Cornuside
Catalog No.:BCN5007
CAS No.:131189-57-6
- PF 5081090
Catalog No.:BCC6148
CAS No.:1312473-63-4
- Antibiotic PF 1018
Catalog No.:BCN2149
CAS No.:131256-42-3
Controversial alkoxyl and peroxyl radical scavenging activity of the tryptophan metabolite 3-hydroxy-anthranilic acid.[Pubmed:28376401]
Biomed Pharmacother. 2017 Jun;90:332-338.
3-Hydroxy-anthranilic acid (3-OHAA), a tryptophan metabolite produced in the kynurenine pathway, is an efficient antioxidant towards peroxyl radicals (ROO) derived from the AAPH (2,2'-azobis(2-amidinopropane) dihydrochloride) thermolysis. However, self-reactions of ROO can give rise to alkoxyl radicals (RO), which could strongly affect the fate of scavenging reactions. In the present work, we studied the influence of RO in the scavenging activity of 3-OHAA in three different systems: i) Monitoring of the direct reaction between 3-OHAA and AAPH-derived free radicals (kinetic studies); ii) Evaluation of the protective effect of 3-OHAA on the AAPH-induced consumption of fluorescein; and, iii) Inhibition, given by 3-OHAA, of the AAPH-initiated lipid peroxidation of both, rat brain synaptosomes and homogenate preparations (assessed by chemiluminescence). For such purposes, the fraction of free radicals (f) trapped per 3-OHAA molecule was determined in each system. Kinetic results show that the oxidation of 3-OHAA follows a process dominated by ROO with a zero order kinetic limit in 3-OHAA, and a fraction (fri) equal to 0.88. From the induction times, elicited by 3-OHAA in the kinetic profiles of fluorescein consumption, a fraction (fT) of 0.28 was determined. 3-OHAA also generated induction times in the kinetic profiles of light emission during the AAPH-initiated lipid peroxidation of rat brain synaptosomes and homogenates. From such induction times, fractions of 0.61 and 0.63 were determined for rat brain synaptosomes (fsyn) and homogenates (fhom), respectively. These results show that during the incubation of 3-OHAA and AAPH, a low fraction of ROO self-reacts to generate RO. Nevertheless, when 3-OHAA is employed to protect particular targets, such as fluorescein, rat brain synaptosomes and homogenates, reactions of ROO and/or RO should be considered.
Untargeted metabolite analysis-based UHPLC-Q-TOF-MS reveals significant enrichment of p-hydroxybenzyl dimers of citric acids in fresh beige-scape Gastrodia elata (Wutianma).[Pubmed:28380386]
J Pharm Biomed Anal. 2017 Jun 5;140:287-294.
In order to comprehensively elucidate the chemical biosynthesis process of the beige-scape Gastrodia elata Blume (Wutianma) as a traditional herbal medicines, the untargeted analysis-based UHPLC-PDA-ESI-Q-TOF-MS reveals the metabolites ranging from the skeletons to novel dimers of citric acids in fresh and dried immature/mature stem tubers. Interestingly, two novel types of dimers for citric acids with the anhydride groups at sn-1 and/or sn-5 were discovered in fresh samples. Moreover, the classical mono- versus novel di-mers, and the aglycons versus the glycosides could be easily discriminated by signature fragmentation patterns and some novel adduct ions. The heat map of contents demonstrated more p-hydroxybenzyl metabolites than gastroxyl ones were determined in fresh Wutianma revealing a significant specificity with the lack of the sufficient gastrodin and gastroxyl products in biosynthetic pathway.
Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice.[Pubmed:28377725]
Front Physiol. 2017 Mar 21;8:139.
Excessive consumption of diets high in sugars and saturated fat, frequently known as western diet (WD), may lead to obesity and metabolic syndrome. Recent evidence shows that WD-induced obesity impairs cardiac function, but the underlying mechanisms are not fully understood. Trimethylamine N-oxide (TMAO), a gut microbiota-dependent metabolite of specific dietary nutrients, has emerged as a key contributor to cardiovascular disease pathogenesis. We tested the hypothesis that elevated circulating TMAO levels contribute to cardiac dysfunction in WD-induced obesity. CD1 mice were fed a normal diet (ND) or a WD, without or with 1.0% 3,3-Dimethyl-1-butanol (DMB, an inhibitor of trimethylamine formation) in drinking water for 8 weeks. Compared with mice fed a ND, mice fed a WD showed a significant increase in body weight and dyslipidemia, and had markedly higher plasma TMAO levels at the end of the feeding protocol. Echocardiography revealed that cardiac systolic and diastolic function was impaired in mice fed a WD. DMB treatment had no effects on body weight and dyslipidemia, but significantly reduced plasma TMAO levels and prevented cardiac dysfunction in mice fed a WD. In addition, mice fed a WD had elevated expression of pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin IL-1beta, decreased expression of anti-inflammatory cytokine IL-10, and increased interstitial fibrosis in the hearts, all of which were prevented by DMB treatment. Notably, DMB treatment also reduced plasma TMAO levels in mice fed a ND but did not alter other parameters. These results suggest that consumption of a WD increases circulating TMAO levels, which lead to cardiac inflammation and fibrosis, contributing to cardiac dysfunction. Interventions that reduce circulating TMAO levels may be a novel therapeutic strategy for prevention and treatment of WD-induced cardiac dysfunction.
Metabolite quantification by NMR and LC-MS/MS reveals differences between unstimulated, stimulated, and pure parotid saliva.[Pubmed:28380387]
J Pharm Biomed Anal. 2017 Jun 5;140:295-300.
Saliva is a readily available biofluid that is sensitive to metabolic changes and can be collected through rapid and non-invasive collection procedures, and it shows great promise for clinical metabolomic studies. This work studied the metabolite composition of, and the differences between, saliva samples collected by unstimulated spitting/drooling, paraffin chewing-stimulated spitting, and parotid gland suction using targeted nuclear magnetic resonance (NMR) spectroscopy and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) for metabolite quantification. As applied here, these two analytical techniques provide complementary metabolite information and together extend the metabolome coverage with robust NMR quantification of soluble metabolites and sensitive targeted LC-MS/MS analysis of bioactive lipids in specific metabolic pathways. The NMR analysis was performed on ultrafiltrated (3kDa cutoff) saliva samples and resulted in a total of 45 quantified metabolites. The LC-MS/MS analysis was performed on both filtered and unfiltered samples and resulted in the quantification of two endocannabinoids (AEA and PEA) and 22 oxylipins, which at present is the most comprehensive targeted analysis of bioactive lipids in human saliva. Important differences in the metabolite composition were observed between the three saliva sample collection methods, which should be taken into consideration when designing metabolomic studies of saliva. Furthermore, the combined use of the two metabolomics platforms (NMR and LC-MS/MS) proved to be viable for research and clinical studies of the salivary metabolome.


