Ro 25-6981 MaleateNMDA receptor antagonist,potent and selective CAS# 1312991-76-6 |
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1312991-76-6 | SDF | Download SDF |
| PubChem ID | 124080916 | Appearance | Powder |
| Formula | C26H33NO6 | M.Wt | 455.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 61 mg/mL (133.91 mM) H2O : 8.33 mg/mL (18.29 mM; ultrasonic and warming and heat to 45°C) *"≥" means soluble, but saturation unknown. | ||
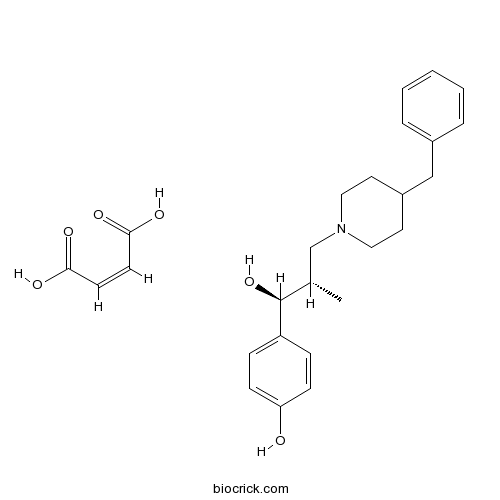
| Chemical Name | 4-[(1S,2S)-3-(4-benzylpiperidin-1-yl)-1-hydroxy-2-methylpropyl]phenol;(Z)-but-2-enedioic acid | ||
| SMILES | CC(CN1CCC(CC1)CC2=CC=CC=C2)C(C3=CC=C(C=C3)O)O.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | FYJZEHCQSUBZDY-YTDXNXAASA-N | ||
| Standard InChI | InChI=1S/C22H29NO2.C4H4O4/c1-17(22(25)20-7-9-21(24)10-8-20)16-23-13-11-19(12-14-23)15-18-5-3-2-4-6-18;5-3(6)1-2-4(7)8/h2-10,17,19,22,24-25H,11-16H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1-/t17-,22-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective activity-dependent blocker of NMDA receptors containing the NR2B subunit. IC50 values are 0.009 and 52 μM for cloned receptor subunit combinations NR1C/NR2B and NR1C/NR2A respectively. Displays neuroprotectant effects in vivo and in vitro. |

Ro 25-6981 Maleate Dilution Calculator

Ro 25-6981 Maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1952 mL | 10.976 mL | 21.952 mL | 43.9039 mL | 54.8799 mL |
| 5 mM | 0.439 mL | 2.1952 mL | 4.3904 mL | 8.7808 mL | 10.976 mL |
| 10 mM | 0.2195 mL | 1.0976 mL | 2.1952 mL | 4.3904 mL | 5.488 mL |
| 50 mM | 0.0439 mL | 0.2195 mL | 0.439 mL | 0.8781 mL | 1.0976 mL |
| 100 mM | 0.022 mL | 0.1098 mL | 0.2195 mL | 0.439 mL | 0.5488 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ro 25-6981 Maleate is a high-affinity, potent and selective blocker of N-methyl- D-aspartate (NMDA) receptors [1].
Ro 25-6981 Maleate has shown a biphasic concentration-effect relationship in inhibiting 3H-MK-801 binding to rat forebrain membranes with the mean Kd values of 0.003μM and 149μM for the high-affinity binding and the low-affinity binding, respectively. In addition, the IC50 values of Ro 25-6981 for antagonism of the NR1C & NR2B, NR1F &NR2B and NR1C & NR2A receptors are 0.009μM, 0.017μM and 52μM, respectively. Moreover, Ro 25-6981 has been revealed to protect the neurons in concentration-dependent fashion in both two toxicity tests with the IC50 values of 0.4μM in the glutamate toxicity test and 0.04μMin the OGD test [1].
References:
[1] Fischer G1, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997 Dec;283(3):1285-92.
- Scutebarbatine Z
Catalog No.:BCN6991
CAS No.:1312716-28-1
- Scutebarbatine Y
Catalog No.:BCN6994
CAS No.:1312716-27-0
- Scutebarbatine X
Catalog No.:BCN6997
CAS No.:1312716-26-9
- Scutebarbatine W
Catalog No.:BCN7011
CAS No.:1312716-25-8
- Antibiotic PF 1018
Catalog No.:BCN2149
CAS No.:131256-42-3
- PF 5081090
Catalog No.:BCC6148
CAS No.:1312473-63-4
- Cornuside
Catalog No.:BCN5007
CAS No.:131189-57-6
- NSC 624206
Catalog No.:BCC7988
CAS No.:13116-77-3
- Sodium Demethylcantharidate
Catalog No.:BCN8394
CAS No.:13114-29-9
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- Ro 8-4304 hydrochloride
Catalog No.:BCC7655
CAS No.:1312991-77-7
- threo Ifenprodil hemitartrate
Catalog No.:BCC7508
CAS No.:1312991-83-5
- Nystose
Catalog No.:BCN5397
CAS No.:13133-07-8
- NVP-CGM097
Catalog No.:BCC5395
CAS No.:1313363-54-0
- erythro-Guaiacylglycerol beta-threo-syringylglycerol ether
Catalog No.:BCN7333
CAS No.:1313434-74-0
- Periplocin
Catalog No.:BCN2655
CAS No.:13137-64-9
- Teijin compound 1
Catalog No.:BCC6057
CAS No.:1313730-14-1
- Boc-Trp-OH
Catalog No.:BCC3455
CAS No.:13139-14-5
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- Boc-Ile-OH.1/2H2O
Catalog No.:BCC3406
CAS No.:13139-16-7
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
Inhibition of in vivo [(3)H]MK-801 binding by NMDA receptor open channel blockers and GluN2B antagonists in rats and mice.[Pubmed:26325093]
Eur J Pharmacol. 2015 Nov 5;766:1-8.
N-methyl-D-aspartate (NMDA) receptor antagonists, including open channel blockers and GluN2B receptor subtype selective antagonists, have been developed for the treatment of depression. The current study investigated effects of systemically administered NMDA channel blockers and GluN2B receptor antagonists on NMDA receptor activity in rodents using in vivo [(3)H]MK-801 binding. The receptor occupancy of GluN2B antagonists was measured using ex vivo [(3)H]Ro 25-6981 binding. Ketamine, a NMDA receptor channel blocker, produced a dose/exposure- and time-dependent inhibition of in vivo [(3)H]MK-801 binding that was maximal at ~100%. The complete inhibition of in vivo [(3)H]MK-801 binding was also observed with NMDA receptor channel blockers, AZD6765 (Lanicemine) and MK-801 (Dizocilpine). CP-101,606 (Traxoprodil), a GluN2B antagonist, produced a dose/exposure- and time-dependent inhibition of in vivo [(3)H]MK-801 binding that was maximal at ~60%. Partial inhibition was also observed with other GluN2B antagonists including MK-0657 (CERC-301), EVT-101, Ro 25-6981 and radiprodil. For all GluN2B antagonists tested, partial [(3)H]MK-801 binding inhibition was achieved at doses saturating GluN2B receptor occupancy. Combined treatment with ketamine (10mg/kg, i.p.) and Ro 25-6981(10mg/kg, i.p.) produced a level of inhibition of in vivo [(3)H]MK-801 binding that was similar to treatment with either agent alone. In conclusion, this in vivo [(3)H]MK-801 binding study shows that NMDA receptor activity in the rodent forebrain can be inhibited completely by channel blockers, but only partially (~60%) by GluN2B receptor antagonists. At doses effective in preclinical models of depression, ketamine may preferentially inhibit the same population of NMDA receptors as Ro 25-6981, namely those containing the GluN2B subunit.
Puberty marks major changes in the hippocampal and cortical c-Fos activation pattern induced by NMDA receptor antagonists.[Pubmed:26995729]
Neuropharmacology. 2017 Jan;112(Pt A):181-187.
Non-selective and subunit (GluN2B)-specific N-methyl-d-aspartate receptor (NMDAR) antagonists represent promising alternative antidepressant drugs with fast onset of the therapeutic action. The neuronal activation pattern induced by NMDAR antagonists is well characterized by c-Fos expression analysis only in the adult rodent brain. In contrast, there is little information available regarding their effects during postnatal development. Here we performed a systematic c-Fos brain mapping of the non-selective NMDAR antagonist MK-801 and the GluN2B-specific antagonist Ro 25-6981 from postnatal day 16 (P16) to P40. We found significant regional differences with gender-specificity in the activation pattern compared to the adult. Surprisingly, in the hippocampus, MK-801 triggered at pre-pubertal stages (especially at P24) very strong c-Fos expression, followed by low levels after P30, the approximate time point of puberty onset in mice. The cortical distribution of MK-801-triggered c-Fos expression before puberty differed also substantially from the adult brain, showing high levels only in deep cortical layers at pre-pubertal stages. In comparison, the cortical activation induced by Ro 25-6981 diminished from high pre-pubertal levels and was in comparison with that triggered by MK-801 low in the hippocampus. These results reveal highly dynamic changes in the c-Fos activation pattern induced by NMDAR antagonists during puberty. This article is part of the Special Issue entitled 'Ionotropic glutamate receptors'.
Different action of a specific NR2B/NMDA antagonist Ro 25-6981 on cortical evoked potentials and epileptic afterdischarges in immature rats.[Pubmed:25446739]
Brain Res Bull. 2015 Feb;111:1-8.
Ro 25-6981 Maleate is a highly selective and activity-dependent antagonist of NMDA ionotropic glutamate receptors containing NR2B subunit (NR2B/NMDARs). The aim of our study was to investigate the influence of Ro 25-6981 administration in developing rats on physiological (single and paired pulse cortical interhemispheric evoked potentials) and epileptic brain activity (cortical afterdischarges (ADs)). Electrophysiological experiments were performed in animals with epidurally implanted electrodes at postnatal days (P) P12, P18, and P25. The drug was injected intraperitoneally at a dose of 1 or 3mg/kg. Control animals were injected with saline (1ml/kg). Single interhemispheric responses were evoked with 0.5-ms biphasic pulses with intensities increasing from 0.4 to 5mA, paired-pulse responses were elicited by twofold threshold intensity. The ADs were elicited by series of 15-s of 1-ms pulses at 8-Hz frequency. Firstly, six stimulations with stable suprathreshold intensity repeated at 30-min intervals were used to determine the time course of Ro 25-6981 effects against ADs in P12 animals. Secondly, similar experiment was performed in all age groups of animals but with 20-min intervals as well as a further experiment using stimulations with stepwise intensities increasing at 10-min intervals from 0.2 to 15 mA. Pretreatment with the 3-mg/kg (but not the lower) dose of Ro 25-9681 decreased significantly the amplitude of single responses evoked with higher stimulation intensities in P12 and P18 animals. Both doses affected responses in P25 animals, only the 1-mg/kg dose was more efficacious than the 3-mg/kg one. Paired pulse responses were not affected by either dose of Ro 25-6981 in any age group. Ro 25-9681 clearly influenced the duration of ADs only in P12 animals. The 1-mg/kg dose did not change the duration of ADs whereas the 3-mg/kg dose suppressed progressive prolongation of ADs with repeated stimulations. This effect was seen even 110-min after the drug injection. The modification of ADs, i.e. stimulations with stepwise increasing intensities (10 min intervals) was used to demonstrate possible dependence on activity. The Ro 25-6981 was administered immediately after the 4-mA stimulation (i.e. when rats experienced six ADs on the average). The 3-mg/kg dose resulted in shorter ADs after high stimulation intensities in P12. There were no significant effects in older animals, only a tendency to ADs shortening was observed in P25 rats. In conclusion, our results indicate that Ro 25-6981 as a selective antagonist of NR2B/NMDARs exhibit age- and activation-dependent anticonvulsant action at early postnatal development. In contrast, the influence of Ro 25-6981 on physiological excitability induced by single pulse stimulation of sensorimotor cortex does not depend on age. This compound may thus represent a useful antiepileptic agent in immature brain since its action against ADs prolongation can be observed even 110 min after the single administration of the drug.
Prefrontal cortical GABAergic and NMDA glutamatergic regulation of delayed responding.[Pubmed:27678413]
Neuropharmacology. 2017 Feb;113(Pt A):10-20.
NMDA glutamatergic and GABAergic transmission have both been implicated in regulating working memory functions mediated by the prefrontal cortex (PFC), and perturbations in these neurotransmitter systems have been proposed to underlie deficits in these functions observed in schizophrenia. Here, we examined the consequence of disrupting GABAergic or NMDA glutamatergic transmission within the medial PFC of rats on a delayed-response paradigm with translational relevance to working memory tasks used with humans. The operant delayed non-match to position task consisted of a sample phase (one lever extended) and a choice phase wherein rats were required to choose the opposite lever, separated by a variable delay (1-24 s). In well-trained rats, inactivation of the PFC via infusions of GABA agonists baclofen/muscimol (100 ng each) induced delay-independent deficits. Reducing PFC GABA transmission with the GABA-A receptor antagonist bicuculline (12.5-50 ng) also caused delay-independent impairments and increased trial omissions and response latencies during the sample and end-of-delay phases. On the other hand, non-selective blockade of PFC NMDA receptors with MK-801 (3-6 mug) disrupted performance, but these effects more closely resembled delay-dependent impairments. However, selective blockade of GluN2B-containing NMDA receptors with Ro-25-6981 (2.5 mug) did not affect any measures of performance. These results demonstrate that both intact PFC GABA and NMDA receptor signalling are integral for accurate delayed-responding, although they may differentially regulate encoding vs maintenance of information within working memory. Furthermore they suggest that perturbations of both of these neurochemical signals within the PFC may contribute differentially to impairments in working memory observed in schizophrenia.
Effects of NMDA-Receptor Antagonist on the Expressions of Bcl-2 and Bax in the Subventricular Zone of Neonatal Rats with Hypoxia-Ischemia Brain Damage.[Pubmed:27352318]
Cell Biochem Biophys. 2015 Nov;73(2):323-330.
Neonatal hypoxia-ischemia brain damage is an important cause of death by affecting prognosis of neural diseases. It is difficult to find effective methods of prevention and treatment due to the complexity of its pathogenesis. N-methyl-D-aspartate (NMDA), as an excitotoxicity amino acids, has proven to play an important role in hypoxic-ischemic. However, the exact effects of the NMDA subunits, NR2A and NR2B, during hypoxic-ischemic have not been investigated in detail. Therefore, we sought to study whether the NMDA receptor antagonist could confer neuroprotective effects in a neonatal rat hypoxia-ischemia model. The effects of intraperitoneal injections of different drugs, namely MK-801 (0.5 mg/kg), NVP-AAM077 (5 mg/kg), and Ro25-6981 (5 mg/kg), on the expressions of anti-apoptotic protein Bcl-2 and apoptosis protein Bax in the subventricular zone were analyzed by immunohistochemical staining to explore the roles of NMDA subunits (NR2A and NR2B) in hypoxic-ischemic. We found that the NR2B antagonist (Ro25-6981) could inhibit hypoxic-ischemic with the increasing Bcl-2 expression. NR2A antagonists (NVP-AAM077) can increase cerebral hypoxia-ischemia in neonatal rats, promoting the expression of apoptotic protein Bax.
The NR2B-selective N-methyl-D-aspartate receptor antagonist Ro 25-6981 [(+/-)-(R*,S*)-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidine propanol] potentiates the effect of nicotine on locomotor activity and dopamine release in the nucleus accumbens.[Pubmed:15256539]
J Pharmacol Exp Ther. 2004 Nov;311(2):560-7.
It has been proposed that nicotine-stimulated locomotor activity (LMA) and nicotine-induced dopamine (DA) release in the mesocorticolimbic DA system is partly regulated by glutamate receptors, particularly N-methyl-D-aspartate (NMDA) receptors. The functional characteristics of NMDA receptors depend on their subunit composition (NR1 in combination with NR2A-D). In the present study, we investigated the effect of the NR2B-selective NMDA receptor antagonist Ro 25-6981 [(+/-)-(R*,S*)-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidine propanol] on nicotine-stimulated LMA and nicotine-induced DA release in the nucleus accumbens (NAcc) in rats. Ro 25-6981 (3 and 10 mg/kg i.p.) given 10 min prior to a high dose (0.6 mg/kg s.c.) or a subthreshold dose (0.1 mg/kg s.c.) of nicotine potentiated nicotine-stimulated LMA with no effect when administered alone. Similarly, administration of a low dose (0.05 mg/kg i.p.) of the noncompetitive NMDA receptor antagonist MK-801 (dizocilpine maleate) had no effect on LMA by itself but potentiated nicotine-induced (0.1 mg/kg) LMA. However, pretreatment with the competitive NMDA receptor antagonist CGP39551 [(E)-(+/-)-2-amino-4-methyl-5-phosphono-3-pentenoic acid ethyl ester] (10 mg/kg i.p.) did not potentiate the LMA effect of 0.1 mg/kg nicotine as seen with Ro 25-6981. In vivo microdialysis revealed a significant increase of DA release in the NAcc in response to nicotine (0.1 mg/kg s.c.). In analogy to our LMA data, Ro 25-6981 (10 mg/kg i.p.) significantly potentiated the nicotine-induced DA release, although it had no effect on DA release when given alone. The data suggest that, compared with other subunits of the NMDA receptor, the NR2B subunit might play a different role in the reinforcing effects of nicotine.
Pharmacological characterization of interactions of RO 25-6981 with the NR2B (epsilon2) subunit.[Pubmed:11290368]
Eur J Pharmacol. 2001 Mar 30;416(3):185-95.
We used ligand binding to ascertain whether the pharmacological actions of RO 25-6981 [(R:(*), S:(*))-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidinepropanol] match those of other NR2B (epsilon2) subunit specific agents. RO 25-6981 inhibited binding of 125I-MK801 [iodo-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohept-5,10-imine maleate] to receptors made from NR1a/epsilon2 but not NR1a/epsilon1. Increasing the concentration of spermidine did not change the efficacy of RO 25-6981 and minimally changed the IC(50) value. Chimeric epsilon1/epsilon2 receptors demonstrated that the structural determinants for high affinity actions of RO 25-6981 were contained completely within the first 464 amino acids, but no receptor retained wildtype features when the size of the epsilon2 component was decreased further. Epsilon1Q336R receptors were more inhibited by ifenprodil and RO 25-9681 than wildtype epsilon1 receptors in ligand binding assays but not in functional assays. Selected mutations of epsilon2E200 and epsilon2E201 also decreased the sensitivity of receptors to ifenprodil and RO 25-6981. These results suggest that RO 25-6981 shares structural determinants with ifenprodil and other modulators in the NR2B subunit.
Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro.[Pubmed:9400004]
J Pharmacol Exp Ther. 1997 Dec;283(3):1285-92.
The interaction of Ro 25-6981 with N-methyl-D-aspartate (NMDA) receptors was characterized by a variety of different tests in vitro. Ro 25-6981 inhibited 3H-MK-801 binding to rat forebrain membranes in a biphasic manner with IC50 values of 0.003 microM and 149 microM for high- (about 60%) and low-affinity sites, respectively. NMDA receptor subtypes expressed in Xenopus oocytes were blocked with IC50 values of 0.009 microM and 52 microM for the subunit combinations NR1C & NR2B and NR1C & NR2A, respectively, which indicated a >5000-fold selectivity. Like ifenprodil, Ro 25-6981 blocked NMDA receptor subtypes in an activity-dependent manner. Ro 25-6981 protected cultured cortical neurons against glutamate toxicity (16 h exposure to 300 microM glutamate) and combined oxygen and glucose deprivation (60 min followed by 20 h recovery) with IC50 values of 0.4 microM and 0.04 microM, respectively. Ro 25-6981 was more potent than ifenprodil in all of these tests. It showed no protection against kainate toxicity (exposure to 500 microM for 20 h) and only weak activity in blocking Na+ and Ca++ channels, activated by exposure of cortical neurons to veratridine (10 microM) and potassium (50 mM), respectively. These findings demonstrate that Ro 25-6981 is a highly selective, activity-dependent blocker of NMDA receptors that contain the NR2B subunit.


