XE 991 dihydrochloridePotent, selective KV7 (KCNQ) channel blocker; blocks M-currents CAS# 122955-13-9 |
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 122955-13-9 | SDF | Download SDF |
| PubChem ID | 45073462 | Appearance | Powder |
| Formula | C26H22Cl2N2O | M.Wt | 449.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
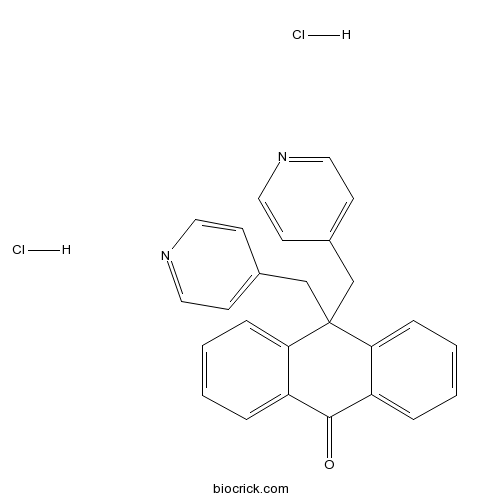
| Chemical Name | 10,10-bis(pyridin-4-ylmethyl)anthracen-9-one;dihydrochloride | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C3=CC=CC=C3C2(CC4=CC=NC=C4)CC5=CC=NC=C5.Cl.Cl | ||
| Standard InChIKey | WOGWMARIFDNZON-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H20N2O.2ClH/c29-25-21-5-1-3-7-23(21)26(17-19-9-13-27-14-10-19,18-20-11-15-28-16-12-20)24-8-4-2-6-22(24)25;;/h1-16H,17-18H2;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective blocker of KV7 (KCNQ) voltage-gated potassium channels. Blocks KV7.2+7.3 (KCNQ2+3) / M-currents (IC50 = 0.6 - 0.98 μM) and KV7.1 (KCNQ1) homomeric channels (IC50 = 0.75 μM) but is less potent against KV7.1/minK channels (IC50 = 11.1 μM). Augments hippocampal ACh release and is a cognitive enhancer following oral administration in vivo. |

XE 991 dihydrochloride Dilution Calculator

XE 991 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2253 mL | 11.1267 mL | 22.2534 mL | 44.5068 mL | 55.6334 mL |
| 5 mM | 0.4451 mL | 2.2253 mL | 4.4507 mL | 8.9014 mL | 11.1267 mL |
| 10 mM | 0.2225 mL | 1.1127 mL | 2.2253 mL | 4.4507 mL | 5.5633 mL |
| 50 mM | 0.0445 mL | 0.2225 mL | 0.4451 mL | 0.8901 mL | 1.1127 mL |
| 100 mM | 0.0223 mL | 0.1113 mL | 0.2225 mL | 0.4451 mL | 0.5563 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-(3-Methoxybenzyl)palmitamide
Catalog No.:BCN8086
CAS No.:847361-96-0
- Fmoc-L-Beta-Homoproline
Catalog No.:BCN8087
CAS No.:193693-60-6
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
- C 21
Catalog No.:BCC8013
CAS No.:1229236-78-5
- GS-9973
Catalog No.:BCC5278
CAS No.:1229208-44-9
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- 3-O-Acetylandrostenone hydrazone
Catalog No.:BCC8637
CAS No.:122914-94-7
- GSK 9027
Catalog No.:BCC6115
CAS No.:1229096-88-1
- Tatarinoid A
Catalog No.:BCN6119
CAS No.:1229005-35-9
- 12-Demethylneocaesalpin F
Catalog No.:BCN6410
CAS No.:1228964-10-0
- MLN0905
Catalog No.:BCC3961
CAS No.:1228960-69-7
- Ziprasidone HCl
Catalog No.:BCC2511
CAS No.:122883-93-6
- URMC-099
Catalog No.:BCC5563
CAS No.:1229582-33-5
- 6'-O-Galloyl paeoniflorin
Catalog No.:BCN2941
CAS No.:122965-41-7
- HA 130
Catalog No.:BCC7884
CAS No.:1229652-21-4
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- Fmoc-Hyp(tBu)-OH
Catalog No.:BCC3256
CAS No.:122996-47-8
- 4-Hydroxybenzaldehyde
Catalog No.:BCN5816
CAS No.:123-08-0
- Anisic aldehyde
Catalog No.:BCN2618
CAS No.:123-11-5
- D-erythro-Sphingosine (synthetic)
Catalog No.:BCC6729
CAS No.:123-78-4
- Azelaic Acid
Catalog No.:BCC8300
CAS No.:123-99-9
- Azasetron HCl
Catalog No.:BCC5035
CAS No.:123040-16-4
- Bulleyanin
Catalog No.:BCN6120
CAS No.:123043-54-9
- Phyltetralin
Catalog No.:BCN3051
CAS No.:123048-17-9
KCNQ/M currents in sensory neurons: significance for pain therapy.[Pubmed:12904483]
J Neurosci. 2003 Aug 6;23(18):7227-36.
Neuronal hyperexcitability is a feature of epilepsy and both inflammatory and neuropathic pain. M currents [IK(M)] play a key role in regulating neuronal excitability, and mutations in neuronal KCNQ2/3 subunits, the molecular correlates of IK(M), have previously been linked to benign familial neonatal epilepsy. Here, we demonstrate that KCNQ/M channels are also present in nociceptive sensory systems. IK(M) was identified, on the basis of biophysical and pharmacological properties, in cultured neurons isolated from dorsal root ganglia (DRGs) from 17-d-old rats. Currents were inhibited by the M-channel blockers linopirdine (IC50, 2.1 microm) and XE991 (IC50, 0.26 microm) and enhanced by retigabine (10 microm). The expression of neuronal KCNQ subunits in DRG neurons was confirmed using reverse transcription-PCR and single-cell PCR analysis and by immunofluorescence. Retigabine, applied to the dorsal spinal cord, inhibited C and Adelta fiber-mediated responses of dorsal horn neurons evoked by natural or electrical afferent stimulation and the progressive "windup" discharge with repetitive stimulation in normal rats and in rats subjected to spinal nerve ligation. Retigabine also inhibited responses to intrapaw application of carrageenan in a rat model of chronic pain; this was reversed by XE991. It is suggested that IK(M) plays a key role in controlling the excitability of nociceptors and may represent a novel analgesic target.
Molecular basis for differential sensitivity of KCNQ and I(Ks) channels to the cognitive enhancer XE991.[Pubmed:10825393]
Mol Pharmacol. 2000 Jun;57(6):1218-23.
Channels formed by coassembly of the KCNQ1 (KvLQT1) subunit and the minK subunit underlie slowly activating cardiac delayed rectifier (I(Ks)) in the heart, whereas two other members of the KCNQ channel family, KCNQ2 and KCNQ3, coassemble to underlie the M current in the nervous system. Because of their important physiological function, KCNQ channels have potential as drug targets, and an understanding of possible mechanisms that would enable tissue-specific targeting of these channels will be of significant value to drug development. In this study, we examined the role of the minK subunit in determining the response of KCNQ1 channels to blockade by the cognitive enhancer XE991. Coexpression with minK markedly decreased the sensitivity of KCNQ1 to blockade by XE991. When measured at the end of a 500-ms step, XE991 blockade of the KCNQ1+minK current had a K(D) value of 11.1 +/- 1.8 microM, approximately 14-fold less sensitive than the block of the KCNQ1 current (K(D) = 0.78 +/- 0.05 microM). In addition, XE991 reduced activation and deactivation time constants and caused a rightward shift in the activation curve of KCNQ1+minK, but affected none of these parameters for KCNQ1 alone. Also, XE991 block of KCNQ1+minK, but not of KCNQ1, was time- and voltage-dependent. We conclude that the presence of minK in the I(Ks) channel complex gives rise to differential sensitivity of KCNQ and I(Ks) channels to blockade by XE991. Our results have implications for drug development by demonstrating the important potential role of accessory subunits in determining the pharmacological properties of KCNQ channels.
KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel.[Pubmed:9836639]
Science. 1998 Dec 4;282(5395):1890-3.
The M-current regulates the subthreshold electrical excitability of many neurons, determining their firing properties and responsiveness to synaptic input. To date, however, the genes that encode subunits of this important channel have not been identified. The biophysical properties, sensitivity to pharmacological blockade, and expression pattern of the KCNQ2 and KCNQ3 potassium channels were determined. It is concluded that both these subunits contribute to the native M-current.
Two new potent neurotransmitter release enhancers, 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone and 10,10-bis(2-fluoro-4-pyridinylmethyl)-9(10H)-anthracenone: comparison to linopirdine.[Pubmed:9580619]
J Pharmacol Exp Ther. 1998 May;285(2):724-30.
Linopirdine (3,3-bis(4-pyridinylmethyl)-1-phenylindolin-2-one, DUP996) is an extensively studied representative of a class of cognition enhancing compounds that increase the evoked release of neurotransmitters. Recent studies suggest that these agents act through the blockade of specific K+ channels. We have recently identified more potent anthracenone analogs of linopirdine: 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone (XE991) and 10,10-bis(2-fluoro-4-pyridinylmethyl)-9(10H)-anthracenone (DMP 543). Although linopirdine possesses an EC50 of 4.2 microM for enhancement of [3H]ACh release from rat brain slices, XE991 and DMP 543 have EC50S of 490 and 700 nM, respectively. In addition to greater in vitro potency relative to linopirdine, both compounds show greater in vivo potency and duration of action. Although 5 mg/kg (p.o.) linopirdine does not lead to statistically significant increases in hippocampal extracellular acetylcholine levels, 5 mg/kg (p.o.) XE991 leads to increases (maximal effect > 90% over baseline) which are sustained for 60 min. Moreover, DMP 543 at 1 mg/kg causes more than a 100% increase in acetylcholine levels with the effect lasting more than 3 hr. At doses relevant to their release-enhancing properties, the only overt symptom consistently observed was tremor, possible via a cholinergic mechanism. These results suggest that XE991 and DMP 543 may prove to be superior to linopirdine as Alzheimer's disease therapeutics. In addition, these agents should be useful pharmacological tools for probing the importance of particular ion channels in the control of neurotransmitter release.


