UvedalinCAS# 24694-79-9 |
Quality Control & MSDS
Number of papers citing our products

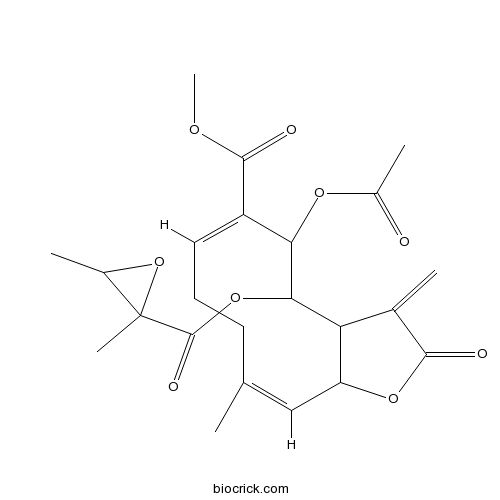
Chemical structure

3D structure
| Cas No. | 24694-79-9 | SDF | Download SDF |
| PubChem ID | 92043370 | Appearance | Powder |
| Formula | C23H28O9 | M.Wt | 448.47 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (6E,10Z)-5-acetyloxy-4-(2,3-dimethyloxirane-2-carbonyl)oxy-10-methyl-3-methylidene-2-oxo-3a,4,5,8,9,11a-hexahydrocyclodeca[b]furan-6-carboxylate | ||
| SMILES | CC1C(O1)(C)C(=O)OC2C3C(C=C(CCC=C(C2OC(=O)C)C(=O)OC)C)OC(=O)C3=C | ||
| Standard InChIKey | JIEVJXRUYDZFTD-XDOJEHJTSA-N | ||
| Standard InChI | InChI=1S/C23H28O9/c1-11-8-7-9-15(21(26)28-6)18(29-14(4)24)19(31-22(27)23(5)13(3)32-23)17-12(2)20(25)30-16(17)10-11/h9-10,13,16-19H,2,7-8H2,1,3-6H3/b11-10-,15-9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Uvedalin shows cytotoxicity against HeLa, HL-60, and Murine B16-F10 melanoma cell lines. |

Uvedalin Dilution Calculator

Uvedalin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2298 mL | 11.149 mL | 22.298 mL | 44.5961 mL | 55.7451 mL |
| 5 mM | 0.446 mL | 2.2298 mL | 4.4596 mL | 8.9192 mL | 11.149 mL |
| 10 mM | 0.223 mL | 1.1149 mL | 2.2298 mL | 4.4596 mL | 5.5745 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.446 mL | 0.8919 mL | 1.1149 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.446 mL | 0.5575 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Senegenin
Catalog No.:BCN5899
CAS No.:2469-34-3
- Derrisisoflavone B
Catalog No.:BCN3955
CAS No.:246870-75-7
- Aglinin A
Catalog No.:BCN5111
CAS No.:246868-97-3
- 1-Benzoyl-4-oxopiperidine
Catalog No.:BCC8455
CAS No.:24686-78-0
- Ro 64-5229
Catalog No.:BCC7513
CAS No.:246852-46-0
- Catharanthine
Catalog No.:BCN1255
CAS No.:2468-21-5
- 3-Amino-2,6-piperidinedione hydrochloride
Catalog No.:BCC8606
CAS No.:24666-56-6
- Neolitsine
Catalog No.:BCN4817
CAS No.:2466-42-4
- Stellasterol
Catalog No.:BCN5110
CAS No.:2465-11-4
- H-Isoleucinol
Catalog No.:BCC2726
CAS No.:24629-25-2
- 3-Geranyl-4-methoxybenzoic acid
Catalog No.:BCN5109
CAS No.:246266-38-6
- AL 8810
Catalog No.:BCC5366
CAS No.:246246-19-5
- Chlorouvedalin
Catalog No.:BCN4664
CAS No.:24694-80-2
- Leonurine hydrochloride
Catalog No.:BCN6286
CAS No.:24697-74-3
- Isomangiferin
Catalog No.:BCN2528
CAS No.:24699-16-9
- NVP DPP 728 dihydrochloride
Catalog No.:BCC2365
CAS No.:247016-69-9
- Weinreb Linker
Catalog No.:BCC2836
CAS No.:247021-90-5
- 7beta-Methoxyrosmanol
Catalog No.:BCN7965
CAS No.:24703-38-6
- 6-Deoxy-9alpha-hydroxycedrodorin
Catalog No.:BCN5112
CAS No.:247036-52-8
- Daphnezomine B
Catalog No.:BCN5113
CAS No.:247078-43-9
- Fimasartan
Catalog No.:BCC5552
CAS No.:247257-48-3
- Dihydroergocristine mesylate
Catalog No.:BCC6657
CAS No.:24730-10-7
- Leonurin monohydrochloride
Catalog No.:BCN8304
CAS No.:24735-18-0
- SB 328437
Catalog No.:BCC6056
CAS No.:247580-43-4
New Sesquiterpene Lactone Dimer, Uvedafolin, Extracted from Eight Yacon Leaf Varieties (Smallanthus sonchifolius): Cytotoxicity in HeLa, HL-60, and Murine B16-F10 Melanoma Cell Lines.[Pubmed:26576855]
J Agric Food Chem. 2015 Dec 23;63(50):10856-61.
Uvedafolin, 1, a new sesquiterpene lactone dimer, was isolated from the leaves of Smallanthus sonchifolius with five related compounds, 2-6, and their cytotoxicity was assessed against three tumor cell lines (HeLa, HL-60, B16-F10 melanoma). The stereostructure of 1 was newly elucidated by ESI-TOF-MS, 1D/2D NMR, and single-crystal X-ray diffraction. Dimers 1 and 2 had the most effective IC50 values, 0.2-1.9 muM, against the three tumor cell lines when compared with monomers 3-6 (IC50 values 0.7-9.9 muM) and etoposide (IC50 values 0.8-114 muM). The ester linkages of two sets of monomers, Uvedalin, 5, and sonchifolin, 6, for 1, and enhydrin, 4, and sonchifolin, 6, for 2, as well as the acetyl group at the C-9 position, were essential for the high cytotoxicity. Dimers 1 and 2 would have potential as anticancer agents.
Trypanocidal Activity of Smallanthus sonchifolius: Identification of Active Sesquiterpene Lactones by Bioassay-Guided Fractionation.[Pubmed:23840260]
Evid Based Complement Alternat Med. 2013;2013:627898.
In order to find novel plant-derived biologically active compounds against Trypanosoma cruzi, we isolated, from the organic extract of Smallanthus sonchifolius, the sesquiterpene lactones enhydrin (1), Uvedalin (2), and polymatin B (3) by bioassay-guided fractionation technique. These compounds showed a significant trypanocidal activity against the epimastigote forms of the parasite with IC50 values of 0.84 mu M (1), 1.09 mu M (2), and 4.90 mu M (3). After a 24 h treatment with 10 mu g/mL of enhydrin or Uvedalin, parasites were not able to recover their replication rate. Compounds 1 and 2 showed IC50 values of 33.4 mu M and 25.0 mu M against T. cruzi trypomastigotes, while polymatin B was not active. When the three compounds were tested against the intracellular forms of T. cruzi, they were able to inhibit the amastigote replication with IC50 of 5.17 mu M, 3.34 mu M, and 9.02 mu M for 1, 2, and 3, respectively. The cytotoxicity of the compounds was evaluated in Vero cells obtaining CC50 values of 46.5 mu M (1), 46.8 mu M (2), and 147.3 mu M (3) and the selectivity index calculated. According to these results, enhydrin and Uvedalin might have potentials as agents against Chagas disease and could serve as lead molecules to develop new drugs.
Effect of different types of sesquiterpene lactones on the maturation of Rhinella arenarum oocytes.[Pubmed:24522008]
Zygote. 2015 Jun;23(3):406-11.
The sesquiterpene lactones (STLs) are a large class of plant secondary metabolites that are generally found in the Asteraceae family and that have high diversity with respect to chemical structure as well as biological activity. STLs have been classified into different groups, such as guaianolides, germacranolides, and melampolides etc., based on their carboxylic skeleton. In amphibians, fully grown ovarian oocytes are arrested at the beginning of meiosis I. Under the stimulus of progesterone, this meiotic arrest is released and meiosis progresses to metaphase II, a process known as oocyte maturation. The purpose of this work was to determine whether sesquiterpene lactones from the germacranolide and melampolide groups act as inhibitor agents on the meiosis of amphibian oocytes in vitro. Results for germacranolides indicated that the addition of deoxyelephantopins caused a high degree of inhibition and that minimolide showed a moderate inhibitory effect, whereas glaucolide A was inactive. Furthermore, the addition of melampolides (Uvedalin, enhydrin, polymatin A and polymatin B) showed inhibitory effects. For enhydrin and Uvedalin, inhibitory effects were observed at the higher concentrations assayed. The results of this study suggest that the inhibitory activity of the tested sesquiterpene lactones on the meiosis of Rhinella arenarum oocytes is not dependent on the group to which they belong, i.e. not on the carboxylic skeleton, but probably due to the arrangement and type of function groups present in the molecules. All assayed lactones in the germacranolide group showed low toxicity. In contrast, important differences in toxicity were observed for lactones from the melampolide group: enhydrin and Uvedalin showed low toxicity, but polymatin A and B were highly toxic.


