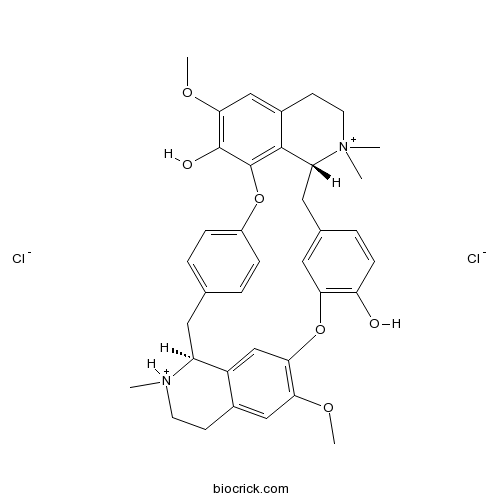
(+)-Tubocurarine chlorideNicotinic receptor antagonist CAS# 57-94-3 |
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- Nexturastat A
Catalog No.:BCC5345
CAS No.:1403783-31-2
- Tubacin
Catalog No.:BCC2428
CAS No.:537049-40-4
- MC1568
Catalog No.:BCC2151
CAS No.:852475-26-4
- Resminostat (RAS2410)
Catalog No.:BCC2165
CAS No.:864814-88-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 57-94-3 | SDF | Download SDF |
| PubChem ID | 64645 | Appearance | Powder |
| Formula | C37H42Cl2N2O6 | M.Wt | 681.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water and to 10 mM in DMSO | ||
| Chemical Name | 2,3,13a,14,15,16,25,25a,-Octahydro- | ||
| SMILES | C[NH+]1CCC2=CC(=C3C=C2C1CC4=CC=C(C=C4)OC5=C6C(CC7=CC(=C(C=C7)O)O3)[N+](CCC6=CC(=C5O)OC)(C)C)OC.[Cl-].[Cl-] | ||
| Standard InChIKey | GXFZCDMWGMFGFL-KKXMJGKMSA-N | ||
| Standard InChI | InChI=1S/C37H40N2O6.2ClH/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33;;/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41);2*1H/t28-,29+;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive, non-selective nicotinic acetylcholine receptor antagonist; causes skeletal muscle relaxation. Also a 5-HT3 and GABAA receptor antagonist. |

(+)-Tubocurarine chloride Dilution Calculator

(+)-Tubocurarine chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.467 mL | 7.3351 mL | 14.6703 mL | 29.3406 mL | 36.6757 mL |
| 5 mM | 0.2934 mL | 1.467 mL | 2.9341 mL | 5.8681 mL | 7.3351 mL |
| 10 mM | 0.1467 mL | 0.7335 mL | 1.467 mL | 2.9341 mL | 3.6676 mL |
| 50 mM | 0.0293 mL | 0.1467 mL | 0.2934 mL | 0.5868 mL | 0.7335 mL |
| 100 mM | 0.0147 mL | 0.0734 mL | 0.1467 mL | 0.2934 mL | 0.3668 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- α-Estradiol
Catalog No.:BCC7497
CAS No.:57-91-0
- Cholesterol
Catalog No.:BCN2199
CAS No.:57-88-5
- Ergosterol
Catalog No.:BCN5787
CAS No.:57-87-4
- Testosterone propionate
Catalog No.:BCC9172
CAS No.:57-85-2
- Progesterone
Catalog No.:BCN2198
CAS No.:57-83-0
- Sulfamethazine
Catalog No.:BCC4942
CAS No.:57-68-1
- Sulfaguanidine
Catalog No.:BCC4727
CAS No.:57-67-0
- Probenecid
Catalog No.:BCC4832
CAS No.:57-66-9
- Ethinyl Estradiol
Catalog No.:BCC3777
CAS No.:57-63-6
- Chlorotetracycline
Catalog No.:BCC8913
CAS No.:57-62-5
- Sucrose
Catalog No.:BCN5780
CAS No.:57-50-1
- Fructose
Catalog No.:BCN4969
CAS No.:57-48-7
- Europine
Catalog No.:BCN1976
CAS No.:570-19-4
- Stigmasta-5,8-dien-3-ol
Catalog No.:BCN5769
CAS No.:570-72-9
- Tricine
Catalog No.:BCN5337
CAS No.:5704-04-1
- Paprotrain
Catalog No.:BCC7978
CAS No.:57046-73-8
- Isosativenediol
Catalog No.:BCN7458
CAS No.:57079-92-2
- 4'-Hydroxywogonin
Catalog No.:BCN5770
CAS No.:57096-02-3
- Boc-D-Lys(2-Cl-Z)-OH
Catalog No.:BCC3421
CAS No.:57096-11-4
- 5α-Androstane-3β,17β-diol
Catalog No.:BCC8751
CAS No.:571-20-0
- 8-Methoxykaempferol
Catalog No.:BCN3344
CAS No.:571-74-4
- Laropiprant
Catalog No.:BCC1688
CAS No.:571170-77-9
- Erastin
Catalog No.:BCC4497
CAS No.:571203-78-6
- 16,17-Dihydroapovincamine
Catalog No.:BCN8049
CAS No.:57130-30-0
Stability of tubocurarine chloride injection at ambient temperature and 4 deg C in polypropylene syringes.[Pubmed:23979307]
Int J Pharm Compd. 2002 Jul-Aug;6(4):308-10.
The stabililty of 3 mg/mL tubocurarine chloride injection in 3-mL polypropylene syringes stored at ambient temperature and at 4 deg C for up to 90 days was investigated. A high-performance liquid chromatographic stability-indicating assay was used to determine concentration levels of tubocurarine chloride injection 0, 1, 4, 7, 15, 30, 45, 60, and 90 days after preparation of the syringes. Benzyl alcohol, which was used as a preservative, did not interfere wiht the assay. At 25 deg C, the loss in potency was less than 10% after the syringes had been stored for 45 days; at 4 deg C, that loss was less than 1% after 90 days of storage. The pH of tubocurarine chloride injection did not change appreciably during the 90-day study period.
Characterization and evaluation of d-(+)-tubocurarine chloride as a chiral selector for capillary electrophoretic enantioseparations.[Pubmed:9529999]
Anal Chem. 1998 Mar 15;70(6):1059-65.
A new macrocyclic of the bis(benzylisoquinoline) alkaloid family, d-(+)-Tubocurarine chloride (DTC), has been evaluated as a chiral selector for the separation of optical isomers of organic carboxylates using capillary electrophoresis (CE). The pertinent physicochemical properties, such as absorption spectrum, isoionic point, and solution conformation, of DTC were determined. The effects of varying such experimental parameters as DTC concentration, pH, and methanol content in the running buffer were assessed. CE separation of the enantiomers of 18 different compounds was achieved using DTC as the chiral selector under optimized background electrolytic conditions.


